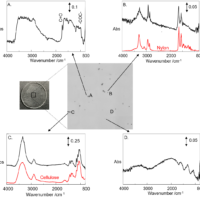
All substances are in a supercritical state above the critical point, but those with a high critical pressure and critical temperature are not practical. Carbon dioxide is a commonly used supercritical fluid, since its critical temperature is just 31.1 degree C and its critical pressure is only 7.38 MPa. It offers the following advantages:
- It is chemically inert and nontoxic.
- It is non-flammable.
- It is non-polar and dissolves oils and fats well.
- Since carbon dioxide is released as a gas at normal temperatures and pressures, solvent removal is easy, allowing more accurate component concentrations to be determined.
- High-purity carbon dioxide can be obtained at a low price, so low running costs can be realized.
- Since carbon dioxide emitted from petrochemical factories is collected, refined and used, it does not increase carbon dioxide emissions.
Video showing the state change of supercritical fluid carbon dioxide (YouTube)