Supercritical fluid chromatography (SFC) uses a supercritical fluid as the mobile phase.
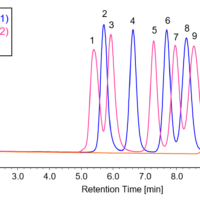
Supercritical fluids are more than an order of magnitude less viscous than liquids, and the diffusion coefficient of substances in supercritical fluids is several hundred times greater than that in liquids. For this reason, SFC can achieve high separation efficiency even when the flow rate of the mobile phase is increased. Therefore, SFC allows substances to be separated at a higher speed than is possible using high-performance liquid chromatography (HPLC) with a liquid as the mobile phase. In addition, the density and properties of supercritical fluids change with pressure and temperature, allowing the separation rate to be adjusted. Furthermore, by mixing an auxiliary solvent (modifier) such as alcohol with the supercritical fluid, it is possible to change the properties of the entire mobile phase and adjust the retention time and separation.
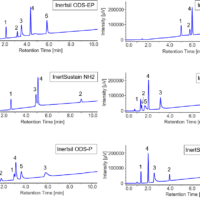
In general, the substance used as the mobile phase in SFC is carbon dioxide. Carbon dioxide has the same polarity as hexane and is suitable for separation of non-polar substances. For this reason, a column with silica gel as a packing material shows retention behavior similar to that for normal phase chromatography. It is also possible to use packed columns for reverse phase chromatography. In this particular case, a modifier is used to separate highly polar substances. In order to separate more highly polar substances, it is necessary to increase the amount of modifier added.
When alcohol is added to carbon dioxide, the critical point of the mixture becomes higher than that for carbon dioxide. For this reason, separation is often performed in subcritical or liquid states where the mobile phase has not reached the supercritical state, but for convenience, it is still referred to as SFC .
Fig. 4 shows the basic flow diagram for SFC. The liquid carbon dioxide is pumped by the liquefied carbon dioxide pump (2). The temperature and pressure are controlled by the column oven (10) and the back-pressure regulator (13) to bring the mobile phase into a supercritical state. If a modifier is added, the modifier pump (5) is used and the composition ratio of carbon dioxide and modifier can be determined arbitrarily. After passing through the column (11), the sample goes to the detector. Since the optical detector is generally placed before the the back-pressure regulator (13), a cell with a high pressure resistance is required.
Fig. 4 Basic flow chart for SFC
Additional components, such as a flame ionization detector, mass spectrometer, and evaporative light scattering detector, can be used with this system. When placing these detectors upstream of the automatic pressure control valve, use the splitter (14) to introduce a portion of the mobile phase into the detector. The solvent can then be added using the ionization accelerator feed pump (16) for the purpose of reliably introducing the mobile phase to the detector and improving the sensitivity. If the flow rate is low, a detector can be placed downstream of the automatic pressure control valve and the entire volume of solution can be introduced.
In semi-preparative / preparative SFC, carbon dioxide is vaporized when the sample is brought to normal pressure, so post-treatment of the solvent after separation is easy.
In some cases, SFC is referred to as unified fluid chromatography or unified chromatography because the mobile phase can be made supercritical, subcritical, or liquid by controlling the pressure, temperature, and modifier ratio.