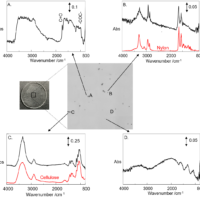
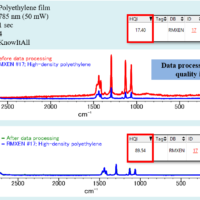
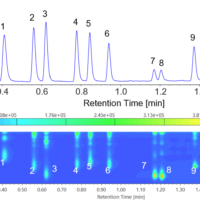
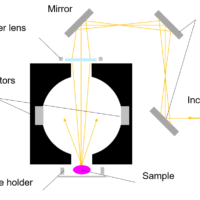
Molecular spectroscopy is a general term for observing a response from molecules interacting with various range of electromagnetic radiation. In this section, some typical methods in molecular spectroscopy utilizing absorption, emission, vibration and scattering are described.
The wave-like nature of electromagnetic radiation can be used in analytical instruments to identify unknown substances and determine their quantity. The field of spectroscopy really began with Newton’s discovery that sunlight could be divided by a prism into a rainbow of seven colors. Although to the human eye, the basic colors appear to be red, yellow, green, blue and violet, plus orange and amber, there are infinite variations between them. Light is electromagnetic radiation, and has both a wave-like and a particle-like nature. When a substance is irradiated with electromagnetic waves, phenomena such as transmission, absorption, reflection and scattering occur.
The period of a wave is known as its wavelength, and an electromagnetic wave is a generic term for waves with wavelengths of 10 km to 10-6 nm. In terms of decreasing wavelength, the sequence is radio waves, microwaves, “light”, X-rays, and γ-rays. In the radio wave region, there are low-frequency waves, ultra-long waves, long waves, medium waves, short waves, ultra-high frequency waves, and microwaves. Radio waves are used for communication, and utilize the fact that they can easily propagate even if there are obstacles in the traveling direction.
The term “light” refers to wavelengths in the range of a few millimeters to a few nanometers. This region of the spectrum is divided into infrared light, visible light, and ultraviolet light in order of decreasing wavelength. X-rays are waves with a wavelength of 1 nm or less, and γ-rays are waves with a wavelength of 10 pm or less. X-rays and γ-rays undergo less interaction with matter than light, and therefore easily penetrate substances. This allows X-rays and γ-rays in particular to be used for imaging. The spectrum that is produced reflects the interaction of waves of a certain wavelength with objects of a certain size, such as atoms, molecules, and polymers.
The spectrum is plotted with the wavelength along the horizontal axis and the intensity along the vertical axis. Since the discovery in the 19th century that light is part of the electromagnetic spectrum, measurements have been performed in various wavelength ranges to obtain spectra that are unique to a particular substance. The spectral intensity is related to the concentration of the substance. This has led to the wide use of spectroscopic techniques for qualitative and quantitative analysis.
The interaction between matter and electromagnetic waves is broadly divided into reflection, transmission and absorption. The waves themselves can undergo scattering, diffraction, interference, and refraction. Electromagnetic energy can be absorbed by phenomena such as chemical reactions, ionization, and the photoelectric effect. Molecular spectroscopy deals with energy absorption by atoms and molecules under irradiation by infrared, visible, or ultraviolet light. Infrared light can be subdivided into far infrared, infrared and near infrared in order of decreasing wavelength. Ultraviolet light can be subdivided into ultraviolet and vacuum ultraviolet in order of decreasing wavelength. The characteristics of light at these different wavelengths are described in “Chapter 3 Light Used in Molecular Spectroscopy”.
In 1932, quantum mechanics revealed that interactions between electromagnetic waves and matter involved the absorption and emission of photons, and spectroscopy became a general term for methods of investigating such interactions, in fields ranging from analytical chemistry to astronomy. In optical or X-ray spectroscopy, waves are dispersed by a prism or a diffraction grating and the wavelength is selected using a slit. On the other hand, analysis methods such as electron spectroscopy and mass spectrometry separate charged particles such as electrons and ions passing through an electromagnetic field according to their kinetic energy. “Dispersing waves by wavelength” and “dividing charged particles by energy” are equivalent to wavelength / frequency / wave number / energy conversion. This is the reason why nuclear magnetic resonance and mass spectrometry are forms of spectroscopy.
Spectroscopy involving radio waves includes submillimeter wave and terahertz spectroscopy. Radio wave spectroscopy has long been applied for global atmospheric and astronomical observations, and has for example been used to investigate atmospheric ozone levels and CO2 convection. Optical spectroscopy includes measurements of absorption, emission, scattering , and photoelectron spectra. Spectroscopic methods using X-rays include fluorescent X-ray methods, X-ray diffraction methods, and X-ray transmission methods. In addition to being used at accelerator research facilities that emit synchrotron radiation, gamma rays are also important in the fields of astronomy and astrophysics.