What is infrared absorption?
In infrared spectroscopy, a sample is irradiated with infrared light, and the transmitted or reflected light is measured, allowing structural analysis and quantification. Absorbance analysis involves measuring the amount of absorption of light by the molecules in a sample, which is wavelength dependent. In the case of ultraviolet and visible light (0.2 to 0.78 μm), absorption occurs due to electronic transitions in molecules. In contrast, infrared light (2.5 to 25 μm) is absorbed due to vibration and rotation of molecules, and the energy involved is smaller (Fig. 1).
Fig. 1 Comparison of infrared and UV/VIS absorption
Molecular vibrations and infrared absorption
In a molecule, interatomic chemical bonds can stretch as if they were springs. The energy associated with such stretching vibrations is close to that of infrared light, so that molecules absorb infrared light and vibrate. The vibrations that can be induced by infrared light are limited to those involving a change in dipole moment. Infrared absorption does not occur if the vibrations of bonds between atoms cancel each other.
In the case of a linear molecule such as CO2 , symmetric stretching vibrations do not absorb infrared light because the dipole moment does not change, but asymmetric stretching vibrations do absorb infrared light because the dipole moment changes. Atmospheric warming occurs because CO2 absorbs infrared light in this manner, and so acts as a greenhouse gas.
In the case of H2O, which is a nonlinear molecule, both symmetric and asymmetric stretching vibrations absorb infrared light because the dipole moment changes. The absorption band due to symmetrical stretching appears at 3,652 cm-1, and that due to asymmetric stretching appears at 3,756 cm-1.
In addition to stretching vibrations, infrared absorption also occurs for bending and rotational vibrations, as long as the dipole moment changes.
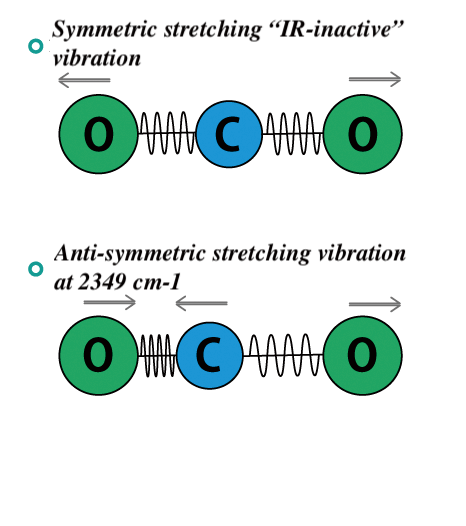
Fig. 2-1 Vibrations in carbon dioxide
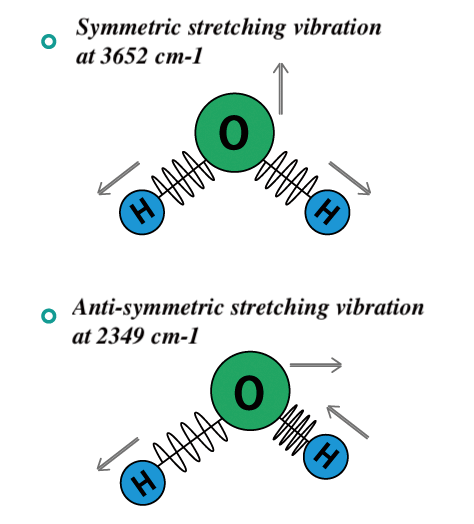
Fig. 2-2 Vibrations in water
Identification of molecules
In an infrared spectrum, absorption peaks associated with functional groups such as -OH and -COOH appear at specific wavenumbers. These regions are referred to as characteristic absorption bands, and the peaks can be analyzed in order to estimate the structure of the compound. The IR spectrum contains information unique to a particular substance, and can be compared with the spectrum of a standard sample stored in a database in order to identify an unknown sample. Hundreds of thousands of standard IR spectra are currently available. These libraries mainly contain information on organic substances, and are used in a wide range of fields such as medicine, agriculture, biology, gas analysis and forensics.
Related Posts:
 Structural Analysis of Chiral Samples with Multiple…
Structural Analysis of Chiral Samples with Multiple… Quantification Method for Oil and Grease in Water…
Quantification Method for Oil and Grease in Water… Combined Analysis of Thermochromic Materials -FT-IR…
Combined Analysis of Thermochromic Materials -FT-IR… Analysis of Hydrogen Bonding in Pure Water using…
Analysis of Hydrogen Bonding in Pure Water using… Analysis of Electronic Structure and Orbital Angular…
Analysis of Electronic Structure and Orbital Angular… Chiral preparative separation of warfarin using…
Chiral preparative separation of warfarin using…
