Far-infrared light

Infrared light with a wavelength of 25 μm to 1 mm. Far-infrared radiation is emitted by all materials, with the intensity depending on the temperature of the object. Far-infrared light causes resonance vibration of the atoms and molecules that constitute a substance, so the temperature of the substance can be raised. Heating appliances such as ceramic heaters are based on this principle. Far-infrared light is also used in thermography, crime prevention sensors, and medical equipment.
Mid-infrared light

Light with a wavelength of 0.7 μm to 1 mm, also referred to as heat radiation. It has a particularly strong thermal effect because its frequency is about the same as the natural vibration frequency of the molecules that make up many substances, leading to a strong resonance effect with no waste. The absorption spectrum can provide clues for determining the chemical composition and molecular structure of a substance.
Near-infrared light

Light with a wavelength of 700 to 3,000 nm, which is between visible light and infrared light. Near-infrared light is generally poorly absorbed because its frequency is higher than the natural vibration frequency of most chemical bonds, and its photon energy is still insufficient to induce electronic transitions. However, since its frequency is close to the overtone frequency of many natural vibrations, weak substance-specific absorption bands can be detected. It can therefore be used for nondestructive measurement of e.g., the sugar content of fruits, and for identifying types of medicine.
Visible light

Light with a wavelength of 380 to 780 nm. This light is scattered by particles. The clear sky looks blue because the shorter-wavelength blue component of sunlight is strongly scattered by nitrogen and oxygen molecules. When the particles are larger than the wavelength of the light, scattering of all visible components occurs. The whiteness of clouds on a sunny day is because of scattering of light by water droplets and ice particles in the clouds.
UV light

Light with a wavelength of 180 to 400 nm. UV light causes significant chemical and physiological changes, and strongly exposes photographic film. Due to its strong bleaching action, pigments and paint are discolored by irradiation by the UV component of sunlight. Sunburn and stains on the skin are also the effects of UV light. It is widely used in industrial processes such as sterilization.
Vacuum UV light
Light with a wavelength of 10 to 190 nm. Vacuum UV light is absorbed by oxygen molecules and propagates only in a vacuum or in an inert gas. Since it decomposes oxygen molecules in the upper atmosphere to form an ozone layer, which serves as a barrier against harmful cosmic UV radiation.
Related Posts:
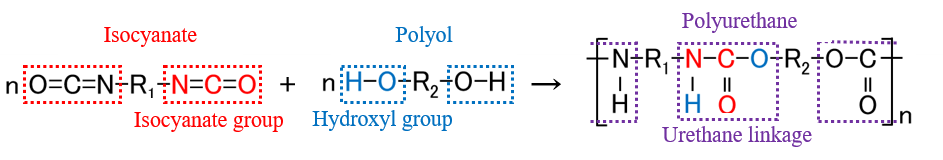 Determining Hydroxyl Value for Polyols Compliant…
Determining Hydroxyl Value for Polyols Compliant… Assessment of Higher Order Structure Similarities…
Assessment of Higher Order Structure Similarities… Evaluation of Higher-Order Structure and Stability…
Evaluation of Higher-Order Structure and Stability…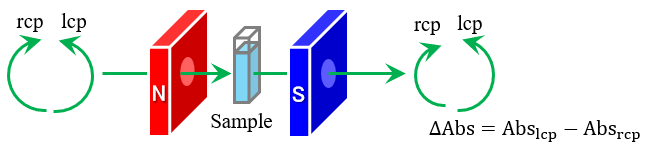 Analysis of Electronic Structure and Orbital Angular…
Analysis of Electronic Structure and Orbital Angular… Multifaceted Analysis of Heat-Degraded Plastics
Multifaceted Analysis of Heat-Degraded Plastics Analysis of electronic structure of phthalocyanine…
Analysis of electronic structure of phthalocyanine…

