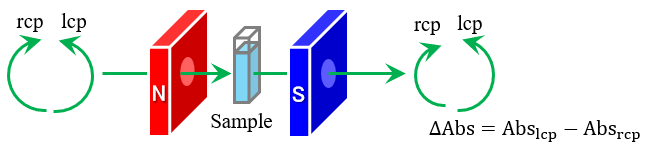
The basis of molecular spectroscopy is the excitation of atoms and molecules by photons. Atoms and molecules excited from the ground state undergo either resonant vibrations or electronic transitions, depending on the nature of the induced quantum mechanical changes. Vibrational transitions correspond to changes in molecular vibrational states and typically appear in the infrared region. Electronic transitions correspond to changes in the electronic state of atoms and molecules, and typically appear in the UV-visible region.
| Vibrational transition | Electronic transition | |||
| Acetaldehyde molecule | Sodium chloride crystal | Carbon atom | Caffeine molecule | Macro molecule |
| Infrared light absorption | Far infrared light absorption | UV light absorption | UV and visible light absorption | UV and visible light absorption |
| Infrared light absorption induces atomic vibrations and rotational movement | Far infrared light absorption causes thermal vibrations of atoms in a solid crystal | UV light absorption by atoms causes electronic transitions to higher energy levels | UV and visible light absorption causes molecular orbital transitions | Light absorption by electrically conducting molecules such as DNA causes electronic transitions from the charge band to the conduction band |
Most types of molecular spectroscopy are referred to as absorption spectroscopy, because they measure the energy loss due to the absorption of photons. Absorption spectroscopy can be divided into vacuum ultraviolet spectroscopy, ultraviolet-visible spectroscopy, near-infrared spectroscopy, infrared spectroscopy, and far-infrared spectroscopy according to the wavelength band used. However, the absorbed energy may also produce radiation due to scattering or light emission. Measurement of such radiation is referred to as emission spectroscopy, which includes techniques such as Raman spectroscopy and fluorescence/phosphorescence spectroscopy.
Both infrared and Raman spectroscopy, which are forms of vibrational spectroscopy, deal with signals derived from molecular vibrations due to infrared irradiation, but different molecular vibrational modes give complementary spectral information.
The figure below shows both the FTIR spectrum (%T) and the Raman spectrum of indoor air. In the Raman spectrum, two sharp peaks corresponding to N2 and O2 molecules are observed, and their intensities reflect the composition of the air. The FTIR spectrum shows vibrational peaks corresponding to H2O (vapor) and CO2. These molecules account for less than 1% of the composition, but each vibrational mode is clearly seen.
Fig. Indoor air spectrum measured by IR (red) and Raman (blue)