What is the Raman effect?
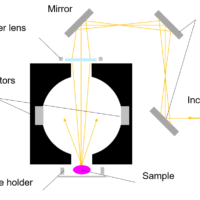
Raman spectroscopy is a popular technique for the analysis of molecular structure and is considered complementary to infrared spectroscopy. Raman spectroscopy is based on the Raman effect, which was first identified by the Indian physicist Chandrasekhara Venkata Raman in 1928. The Raman effect is based on scattering of light, which includes both elastic (Rayleigh) scattering at the same wavelength as the incident light, and inelastic (Raman) scattering at different wavelengths, due to molecular vibrations. Raman scattering is about a million times less intense than Rayleigh scattering. Therefore, to obtain Raman spectra, it is necessary to prevent Rayleigh scattering from overpowering the weaker Raman scattering.
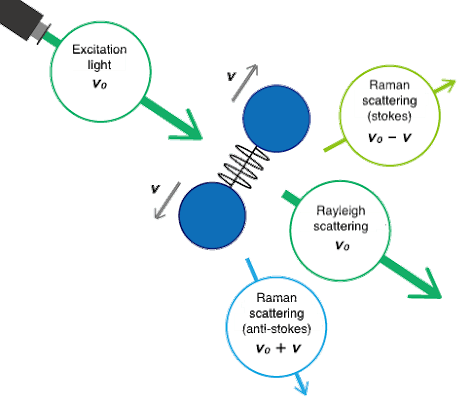
Fig. 1 Molecular vibrations and Raman scattering
Raman spectra are measured by exciting a sample using a high-intensity laser beam, with the resulting scattered light being passed through a spectrometer. The Raman shift is the energy difference between the incident light and the scattered light. In the resulting spectrum, the vertical axis is the intensity of the scattered light and the horizontal axis is the wavenumber of the Raman shift (cm-1).
What is the Raman shift?
The Raman shift is associated with two different energy bands. The shift at wavelengths higher than that of the incident light is termed Stokes scattering. The shift at wavelengths lower than that of the incident light is termed anti-Stokes scattering. As an example, the Raman spectrum of sulfur measured with an excitation wavelength of 532 nm (green laser) is shown in Fig. 2. Stokes scattering is observed in the lower wavenumber (longer wavelength) region and anti-Stokes scattering in the higher wavenumber (shorter wavelength) region. Typically, higher-intensity Stokes scattering peaks are used for analysis, but anti-Stokes peaks can also be used.
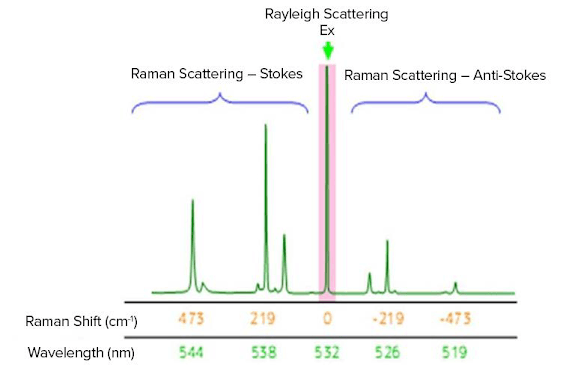
Fig. 2 Raman spectrum of sulfur
Difference between Raman spectroscopy and IR spectroscopy
Both Raman spectroscopy and IR spectroscopy are based on molecular vibrations as illustrated below. Infrared spectroscopy is based on absorption of light energy corresponding to the vibrational energy of molecules. Raman spectroscopy is based on scattering of incident light at an energy shifted by the vibrational energy (hν) of the molecule. Vibration modes for the same functional groups are observed at the same wavenumber.
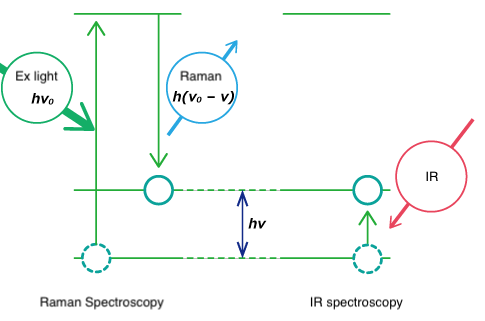
Fig. 3 Vibration energy level
Though both are forms of vibrational spectroscopy, IR and Raman spectroscopy differ in some fundamental aspects, as shown in Fig. 4. IR spectroscopy is based on the fact that molecular absorption at specific vibrational frequencies causes a change in the dipole moment. Raman spectroscopy relies on the change in the polarizability of a molecule at the frequencies (Raman shift) at which the molecule scatters radiation. IR spectroscopy is sensitive to hetero-nuclear functional group vibrations and polar bonds, especially OH stretching in water. Raman spectroscopy is sensitive to homo-nuclear molecular bonds such as C-C, C=C and C≡C bonds.
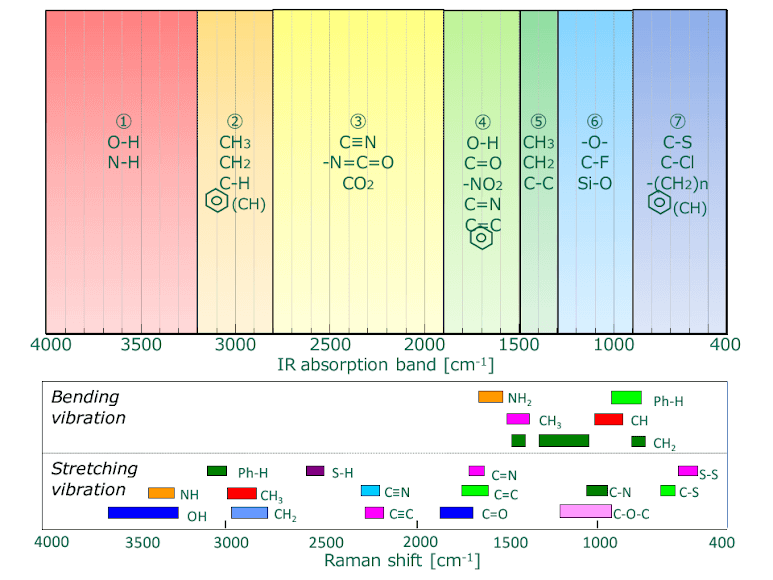
Fig. 4 IR absorption bands (upper) vs. Raman shift (lower) for functional groups
A comparison of IR transmission and Raman spectra for L-cystine is shown in Fig. 5. The intensity of the two spectra exhibit mirror symmetry, so IR and Raman spectra are often considered to be “complementary”. But they are different in the type of physical phenomenon they can measure. In IR measurements, the spectral intensity depends on the size of the dipole moment for vibration modes for bonds such as C=O and O-H. On the other hand, in Raman spectroscopy, the intensity depends on the degree of polarizability (electron volume) for vibration modes for bonds such as S-S, C-C, and CN.
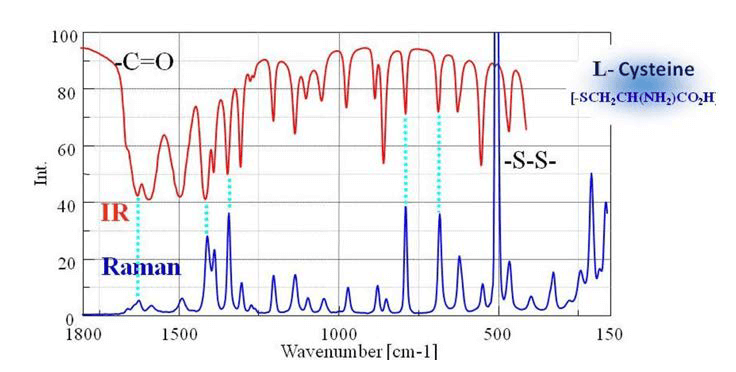
Fig. 5 Comparison of IR and Raman spectra