Stopped-flow measurements involve the rapid mixing of two or more solutions to trigger a chemical reaction, the kinetics of which can be followed by CD, absorbance and fluorescence. All probe methods can be measured on the same instrument when the stopped-flow system is paired with a J-1500 CD spectrometer.
Stopped-flow systems
The SFS-600 series is an innovative stopped-flow measurement accessory with a modular design that allows the flow cell unit to be easily installed and removed from the sample compartment without alignment. Two-, three- and four-syringe models are available, offering flexible mixing as well as upgradeability for quench-flow. For temperature-dependent kinetic experiments, the options are Peltier temperature-controlled syringes. Stepper-motor-driven syringes allow infinitely variable mixing ratios and a mechanical mixer efficiently mixes solutions of different viscosities commonly used in protein folding experiments.
• Applications to studies such as protein folding, substrate binding and enzyme kinetics
• Standard 2 mm cell (optional 0.5, 1 and 10 mm cells)
• Standard 10 mL syringe (optional 1, 2.5, and 5 mL syringes)
• 5 mL/sec. flow rate with 10 mL syringe
• Accurate flow rate control
• Mixing ratio from 1:1 to 1:20
• Dead time: 0.57 msec with a 2 mm cell
• Peltier temperature control (SFS-602T/SFS-603T/SFS-604T) ranges from 5 to 80 °C (with cell) and 5 to 60 °C (with syringe)

Note: The SFS-500 series of stopped-flow accessories is still available for use with the J-1700 CD spectrometer.
Application
Unfolding of Concanavalin A by Trifluoroethanol
Concanavalin A is a lectin protein derived from the jack bean. In its native state, it is composed of abundant beta-sheet structures that change to α-helical form when exposed to Trifluoroethanol (TFE).
The J-1500 and the SFS-602 high-speed stopped-flow system were used to measure the unfolding process of Concanavalin A with TFE.
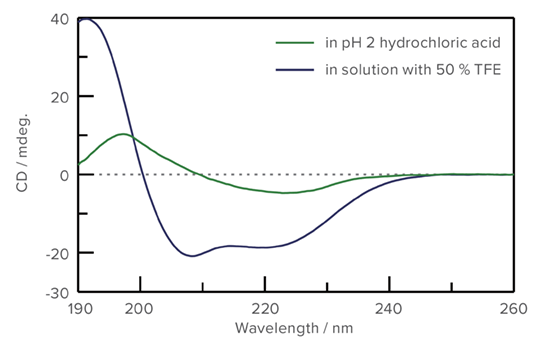
CD Spectra of Concanavalin A
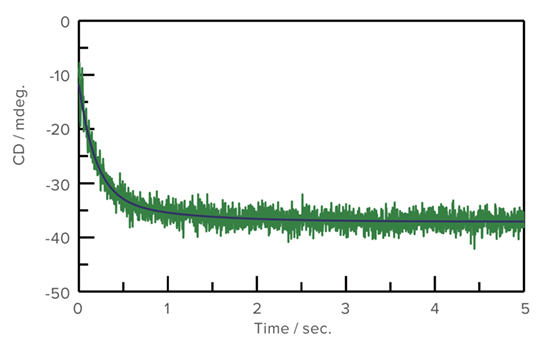
Unfolding process of Concanavalin A in TFE and analysis result
Refolding of Cytochrome C using GuHCl
An aqueous solution of Cytochrome C, which was denatured by Guanidine Hydrocholoride (GuHCl), was prepared. CD stopped-flow measurement was performed using 0.1 M acetic acid buffer/water solution (1:9). The refolding process was observed at both 222 nm for the secondary structure and 289 nm for the environment of the aromatic side chain.
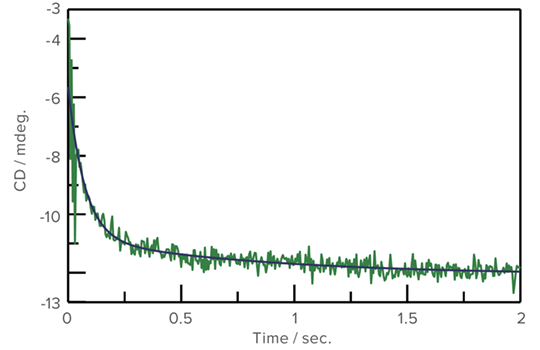
Refolding measurement of Cytochrome C (222 nm)
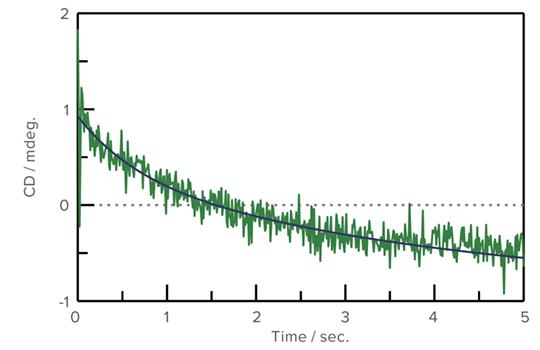
Refolding measurement of Cytochrome C (289 nm)
