Introduction
Biopharmaceuticals have attracted a great deal of attention in recent years since antibody based drugs and protein formulations can target specific regions of the body with few side effects. However, biopharmaceuticals can easily change their structure depending on formulation conditions such as the solvent, pH and temperature, and may consequently lose their activity. It is therefore essential to investigate the effects of formulation conditions in order to ensure quality consistency. CD measurements are extremely effective for structural analysis and stability evaluation of these biopolymers, because structural changes in proteins and nucleic acids are reflected in their CD spectra. Consequently, CD measurements have begun to be utilized during the development phase of biopharmaceuticals, and for identifying the original drug and biosimilars in order to ensure consistent quality. In response to this demand, JASCO has developed a system that can automatically measure the CD spectrum of multiple specimens formulated under different conditions, in addition to software that can quantitatively evaluate CD spectral changes associated with structural changes in proteins. This represents a major advance with widespread applications in the field of biopharmaceuticals.
Here, we report a new approach to evaluating the stability of antibodies by comparing the CD spectra of native and denatured antibodies using statistical analyses before conducting thermal denaturation tests, which generally require a great deal of time.

High-throughput CD system
– Automated sample measurement procedure, including washing and drying the flow path and flow cell – 3 times faster than can be achieved manually!
– Easy, high-sensitivity identification of structural changes in biopharmaceuticals!
– Automatic measurement of up to 192 samples!
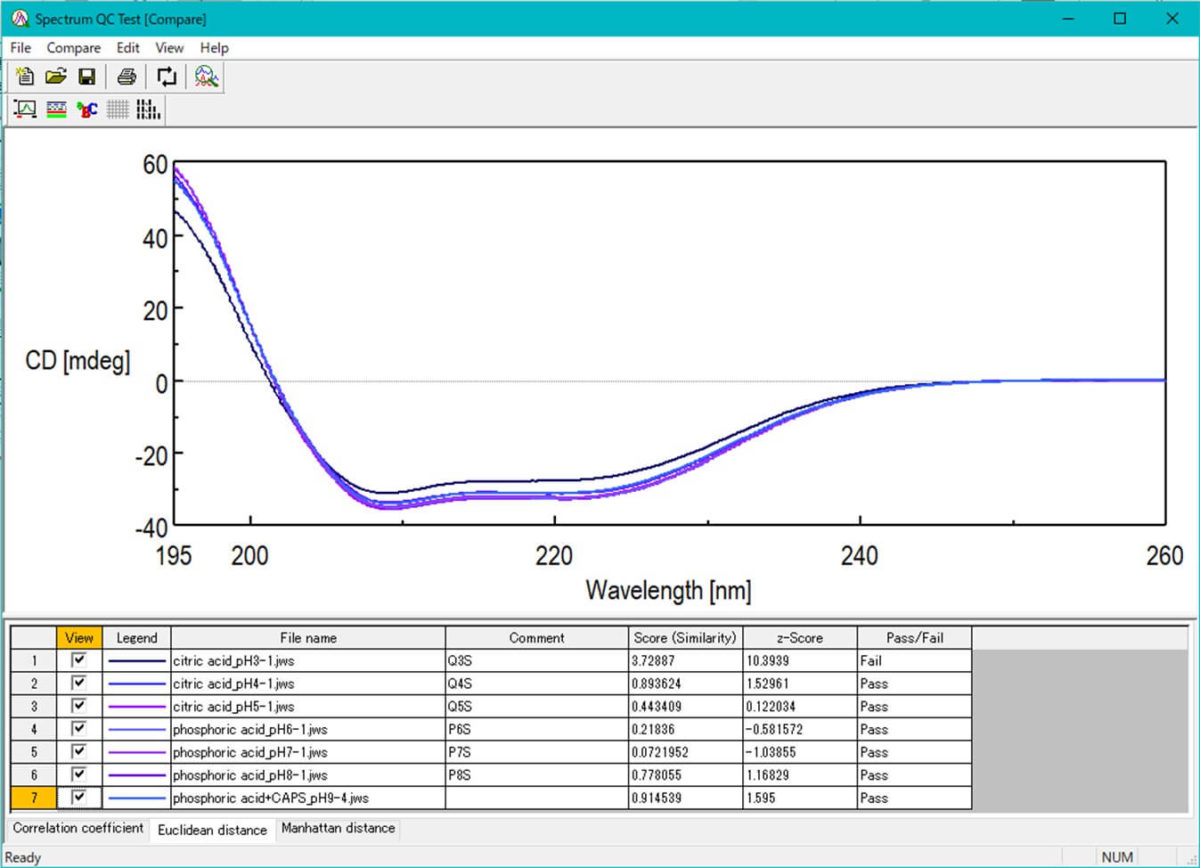
Spectrum QC Test
– Pass/fail decision by quantifying the similarity of CD spectra using a statistical method
– Effective for evaluating the stability and similarity of middle-molecular and polymer drugs
– Small spectral changes can be detected
Experimental
Measurement conditions
– BSA
Spectra measurement
Sample concentration: 1 mg/mL
Cell: 0.2 mm
Reference sample: pH7
– VHH
– VHH has a higher proportion of neutralizing antibodies than normal IgG antibodies.
– Since the molecular weight of VHH is only about 15 KDa, it can easily be expressed.
– VHH easily returns from a denatured state to its natural structure.
– VHH can be fused with proteins and peptides to add new functionality.
Spectra measurement
Sample concentration: 0.5 mg/mL
Cell: 0.2 mm
Reference sample: pH7
Temperature measurement
Sample concentration: 0.5 mg/mL
Cell: 1 mm
Keywords
Antibody, Protein, Screening, BioPharm, Circulardichroism,
Results
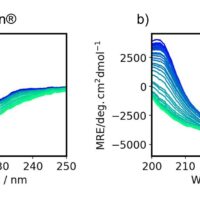
BSA
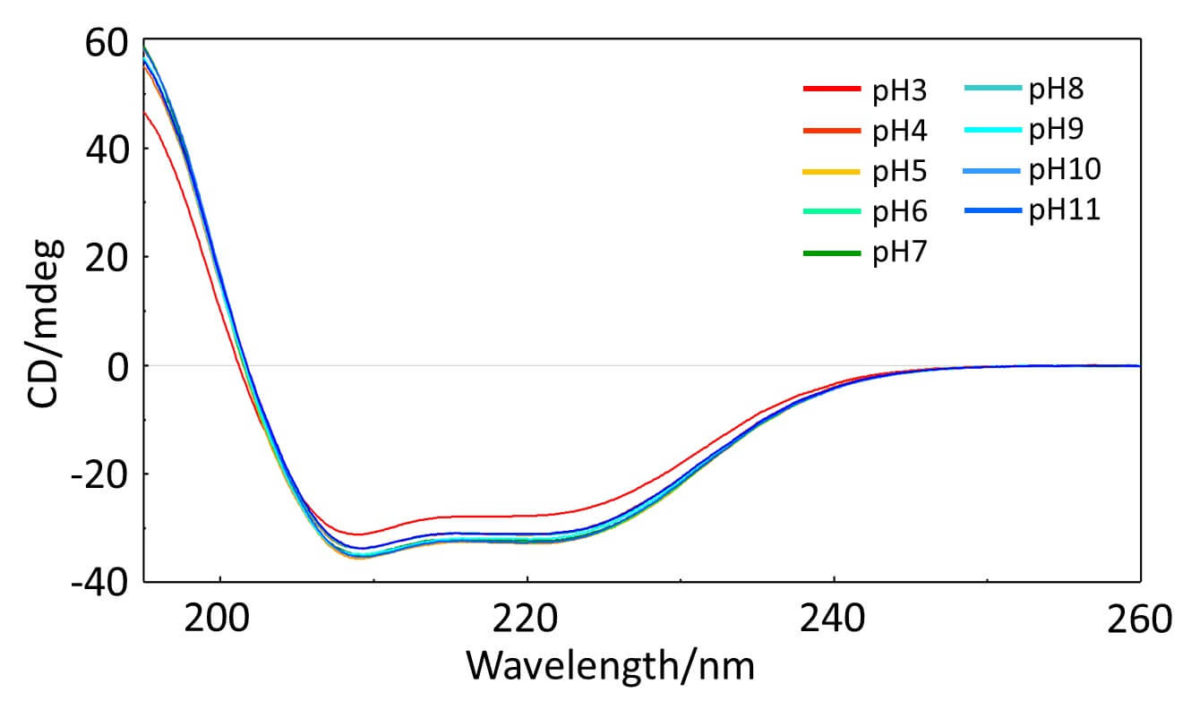
Fig1. pH dependence of CD spectrum of BSA solution
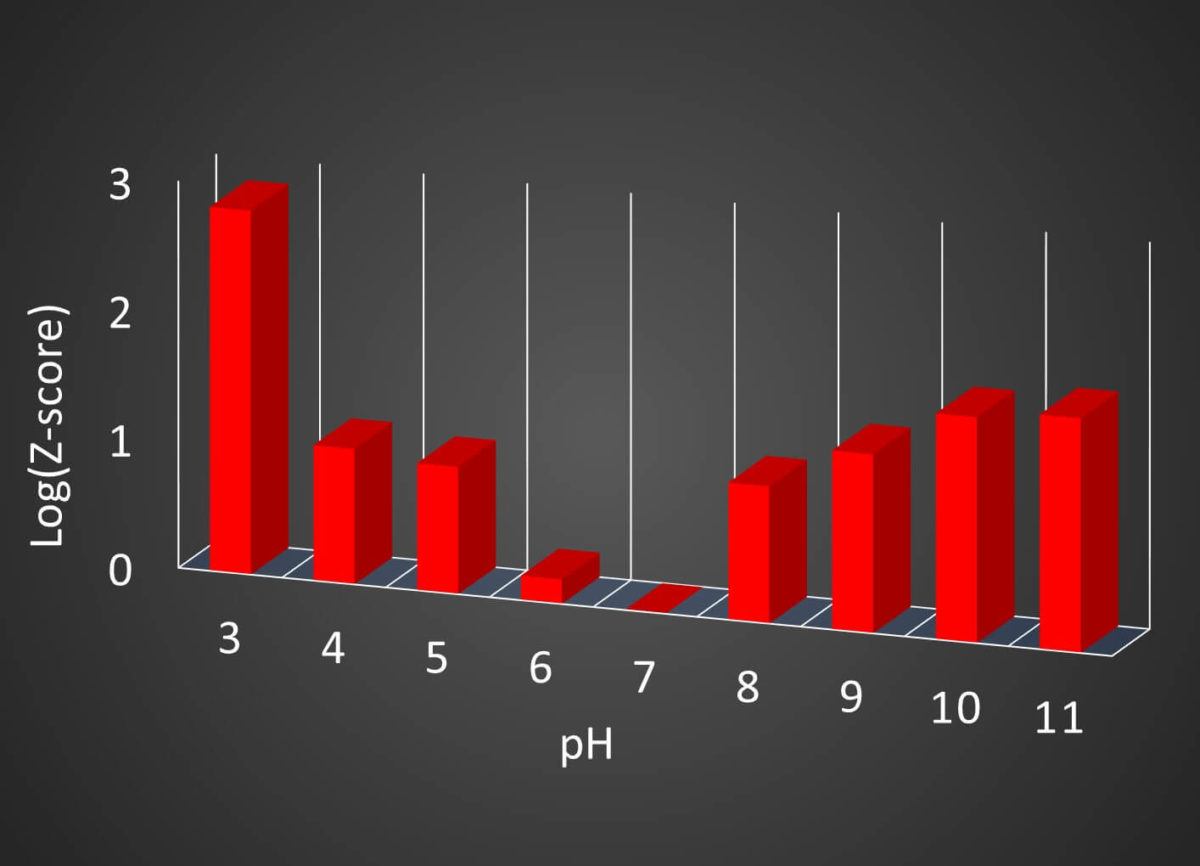
Fig 2. Histogram of log(Z-score) vs. pH
The Z-score shows that the structure of BSA changes as the pH becomes higher (alkaline) or lower (acidic) than 7.
VHH
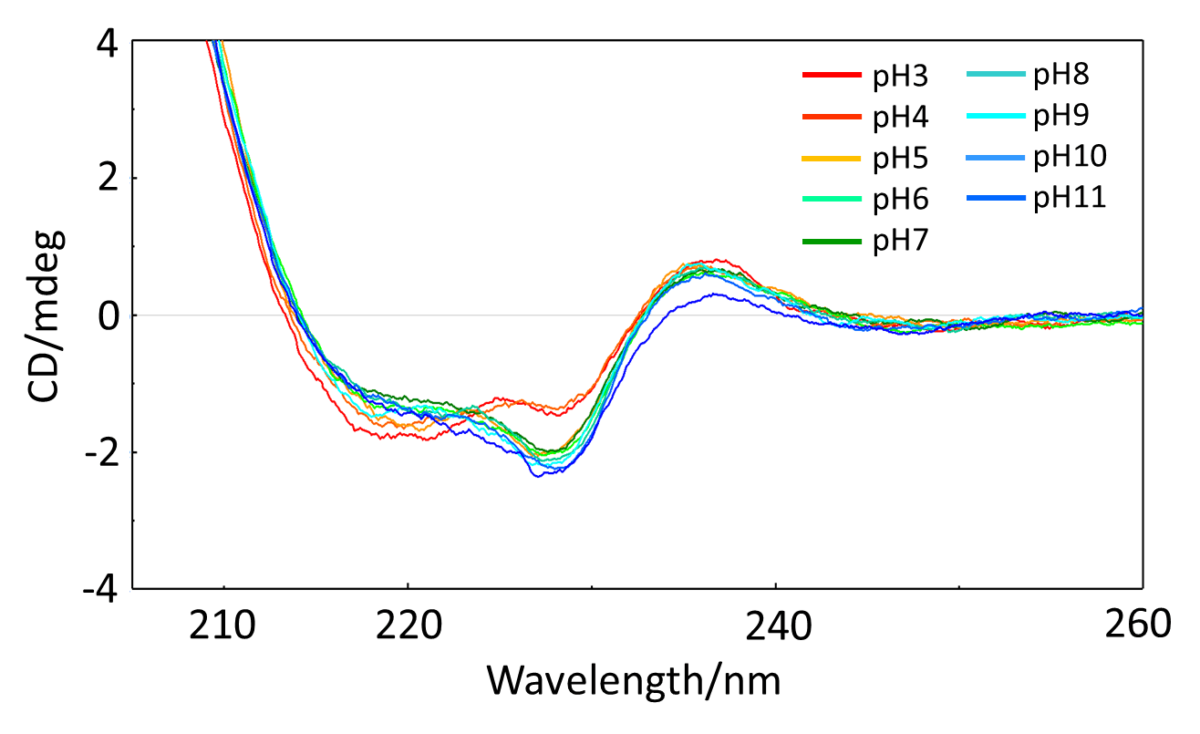
Fig 3. pH dependence of CD spectrum of VHH solution (NaCl conc. 200 mM)
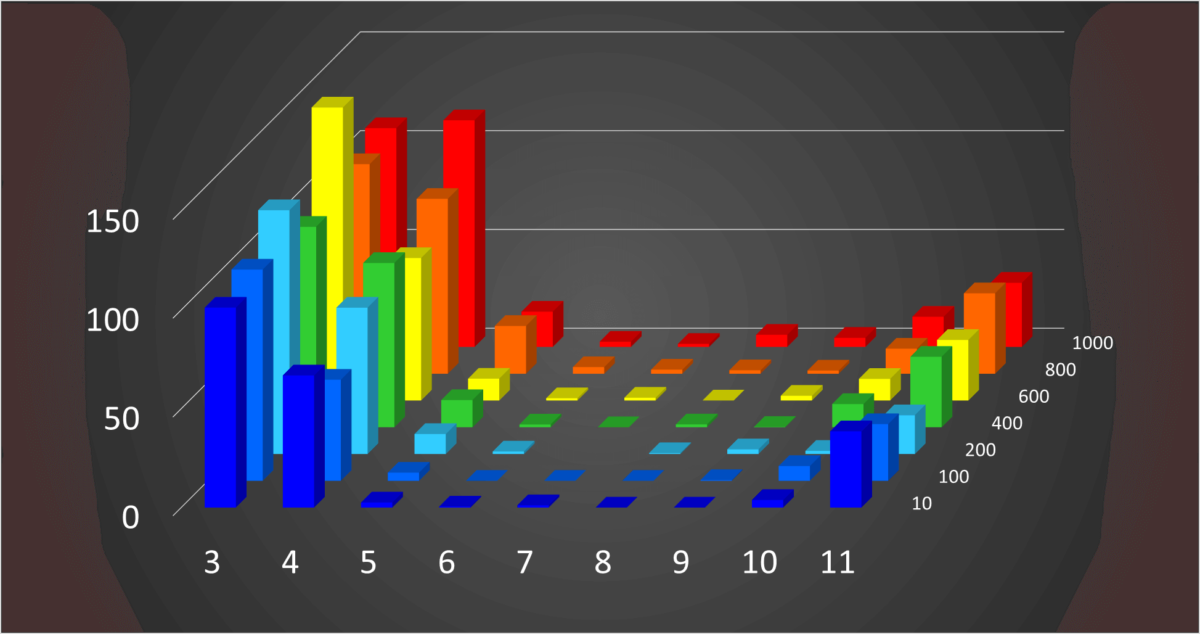
Fig 4. 3-D Histogram of Z-score vs. pH vs. NaCl concentration
The Z-score shows that the structure of VHH changes as the pH becomes higher or lower than 7 and as the NaCl concentration becomes larger than 200 mM.
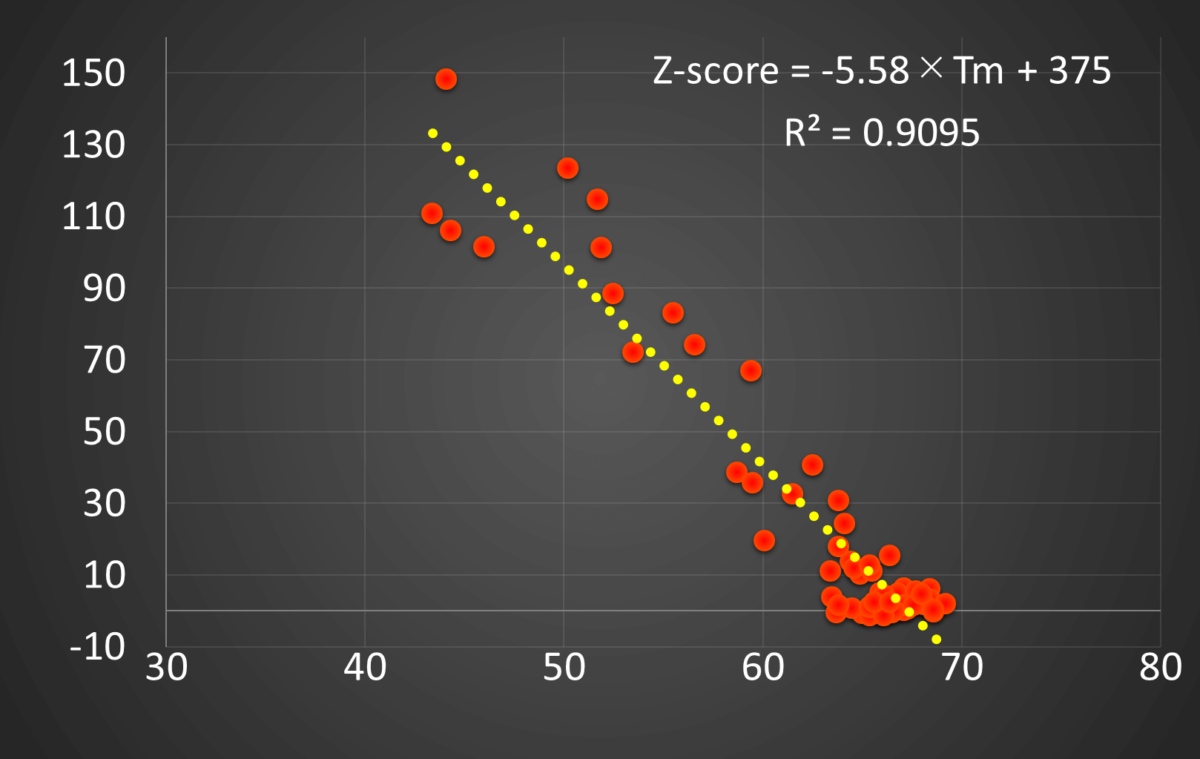
Figure 5. Plot of Z-score vs. Tm
The good correlation between the Z-score and Tm suggests that stability evaluation of antibody proteins can be performed using the Z-score instead of Tm.
Conclusion
The combination of automated high-throughput CD measurements and the Spectrum QC Test software is a breakthrough approach to analyzing the effects of preparation conditions and synthesis methods for biopharmaceuticals.
The high correlation between the Z-score and the denaturation temperature suggests that a Z-test is a very useful primary screening method before conducting a thermal denaturation analysis, which generally requires a great deal of time.
References
Poster session at Biophysical Society meeting 2019.
Satoko Suzuki 1, Yasuo Horiguchi1, Leah Pandiscia2, Koushi Nagamori1, and Kouhei Tsumoto3
1 JASCO Corporation, Tokyo, Japan,
2 JASCO Inc., Maryland, USA,
3 School of Engineering and Institute of Medical Science, The University of Tokyo, Tokyo, Japan