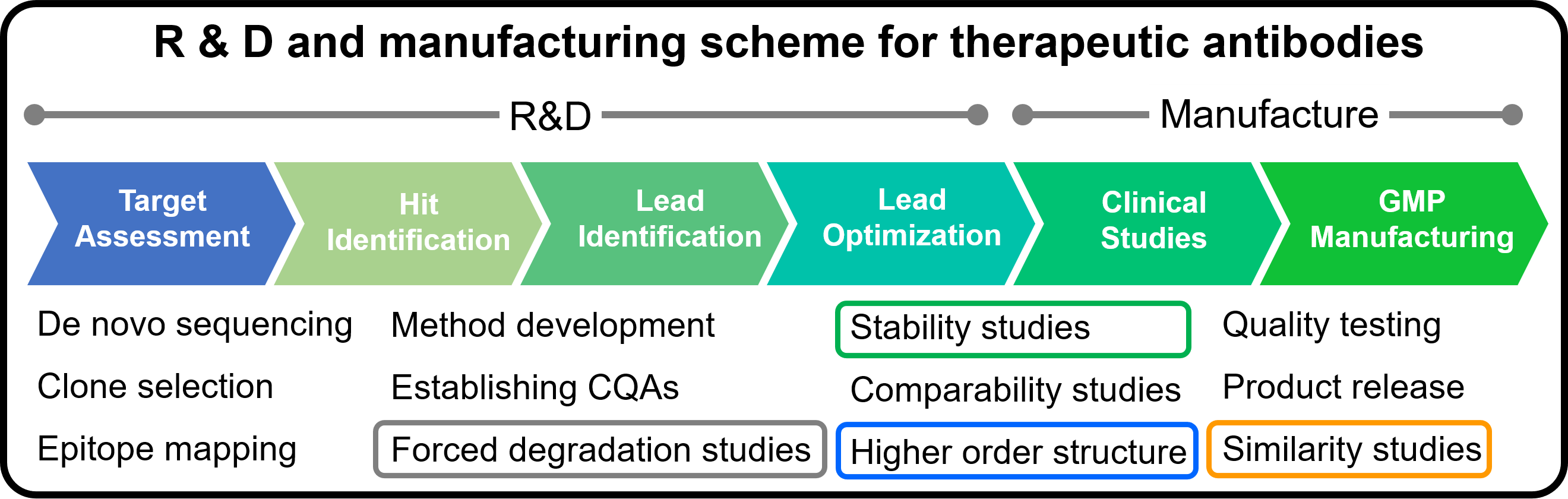
Introduction
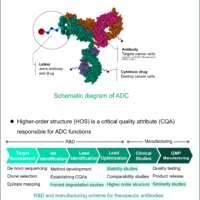
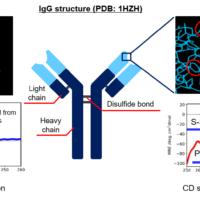
The market for therapeutic antibodies has expanded dramatically over the past several decades. Since antibodies express their activity by forming a higher order structure (HOS), their functions can be significantly impaired by various stimuli, modifications, and degradations during the manufacturing process. Therefore, HOS is one of the Critical Quality Attributes (CQA), and HOS assessment, stability, similarity, and forced degradation studies (FDS) are conducted during the R&D and manufacturing processes. In these assessments, CD spectroscopy is widely used because it allows assessment at low concentrations under homogeneous conditions and in a simple manner.
In this study, we report a case study on HOS assessment, stability and similarity for VHH antibodies, which are attracting attention as a next-generation therapeutics discovery modality following therapeutic antibodies, because of their high affinity, stability, productivity, and processability to antigens.
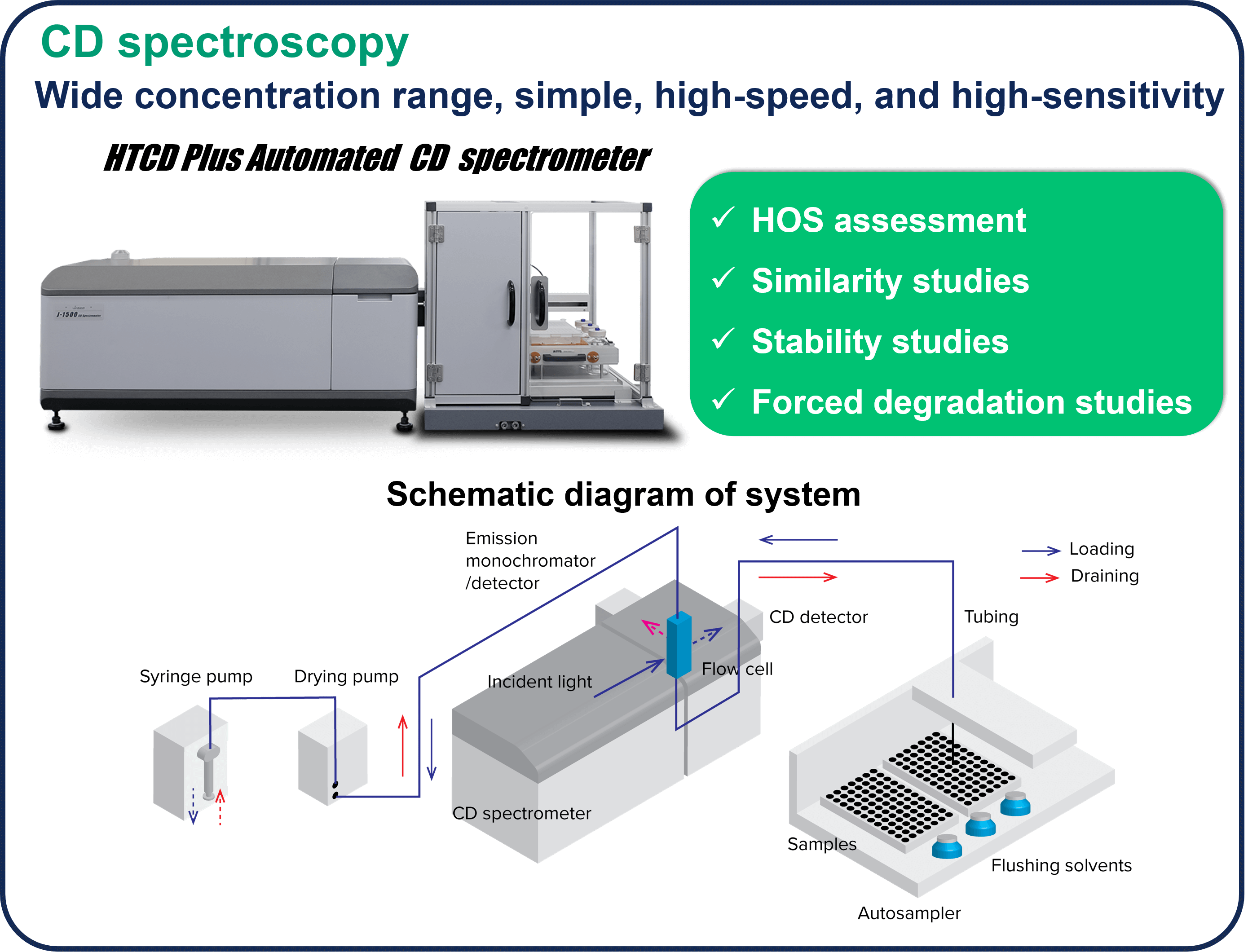
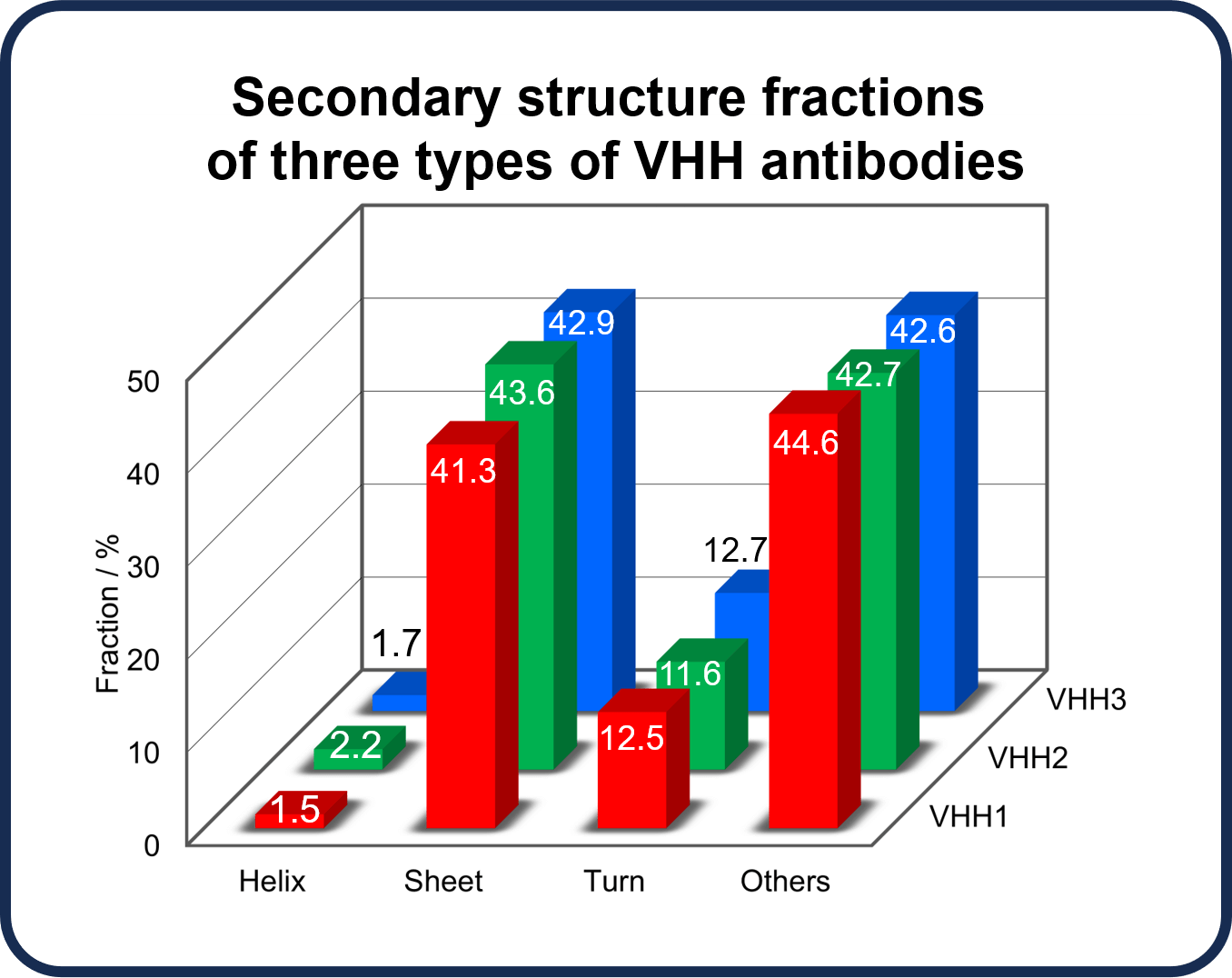
1. HOS assessment
Secondary structure fractions were estimated from CD spectra in the far-UV region for three anti-SARS-Cov-2 VHH antibodies, VHH1, VHH2, and VHH3, with different sequences, provided by RePHAGEN Co., Ltd (https://rephagen.com/en/). This analysis was performed using BeStSel, the world’s highest-performance secondary structure prediction algorithm developed by Dr. József Kardos and Dr. Micsonai András et al. at Eötvös Loránd University. Analysis using BeStSel can be performed offline using JASCO software, or online (https://bestsel.elte.hu).(1-5) The analysis results show that all antibodies have an approximately 40% sheet structure and a 2% helix structure. HOS information for the proteins can thus be easily obtained using CD spectroscopy.
2. Similarity studies
Biosimilars tend to be thought of as having the same function and structure as the innovators because they have the same amino acid sequence. However, antibodies are subjected to various stimuli and post-translational modifications during the production process, which may result in a significant loss of function. Therefore, measuring changes in antibody structure due to stimulation or modification is an essential step in the research and development process, and for quality control of antibody drugs. Guidelines issued by the U.S. Food and Drug Administration (FDA), European Medicine Agency (EMA), and others indicate the importance of analytic measurements of HOS similarity in the quality assessment of biosimilars. CD spectroscopy is widely used as a highly sensitive and simple method for HOS assessment.
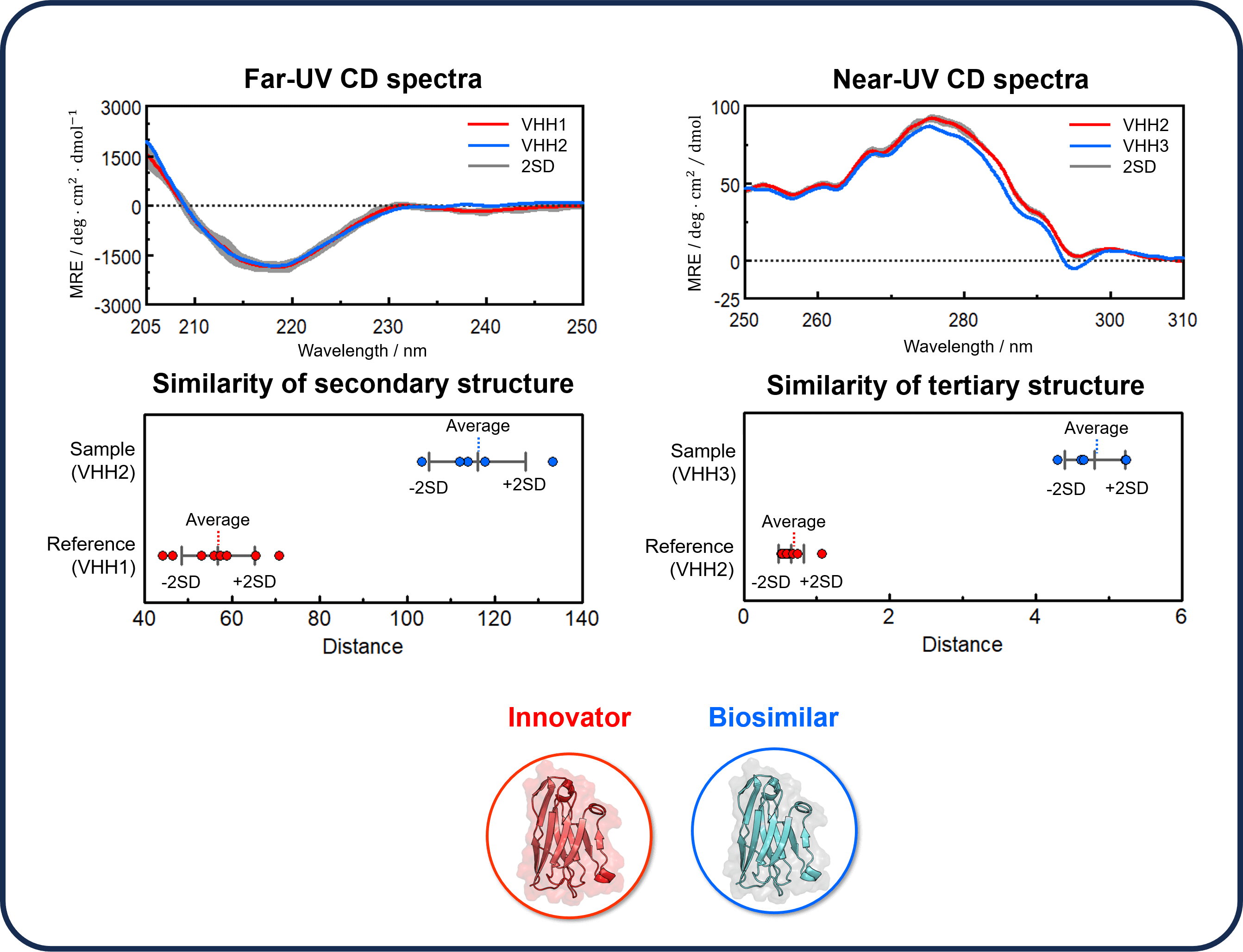
In this study, we used the JASCO qHOS program,(6) which allows HOS similarity assessment by detecting slight spectral differences, to assess the similarity of the CD spectra of VHH antibodies, whose differences cannot be clearly distinguished visually, and to clarify their structural differences. The results showed that the CD spectral shapes for VHH1 and VHH2 in the far-UV region were similar, but their distance distributions were very different. The results of the TOST equivalence test showed that the p-values were below the significance level of 0.05, and the similarity of the secondary structures was not confirmed. The same test was also performed for the CD spectra of VHH2 and VHH3 in the near-UV region. The p-value was below 0.05, and the similarity of the tertiary structure was also not confirmed.
3. Stability studies
It is essential to assess the structural stability of therapeutic antibodies since they may change their structures and become inactivated depending on storage conditions such as temperature, and formulation conditions such as additives and pH. Here, we present a case study to assess the thermal stability of VHH antibodies and their structural stability under different pH and salt concentrations.
3.1 Thermal stability
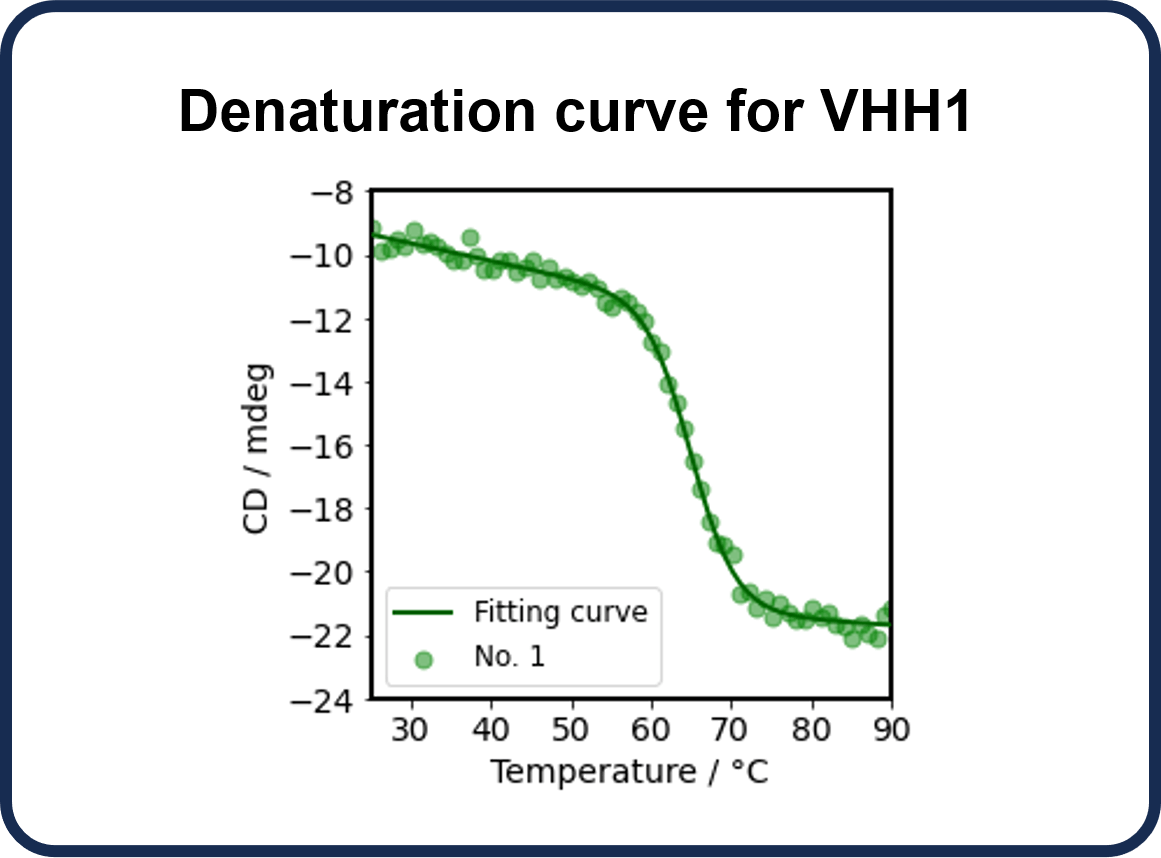
CD spectroscopy can be used to assess thermal denaturation of antibodies. It provides information on the onset (To), denaturation (Tm), and endpoint (Te) temperatures and the refolding ability of the antibody, as well as changes in HOS with temperature. Here, we performed thermal denaturation measurements on VHH1 to determine To, Tm, and Te, and assessed the refolding ability by monitoring changes in the secondary structure fractions using BeStSel while increasing and decreasing the temperature.
The CD values at 217 nm derived from β-sheets showed that To was 57 °C, Tm was 65 °C, and Te was 75 °C.
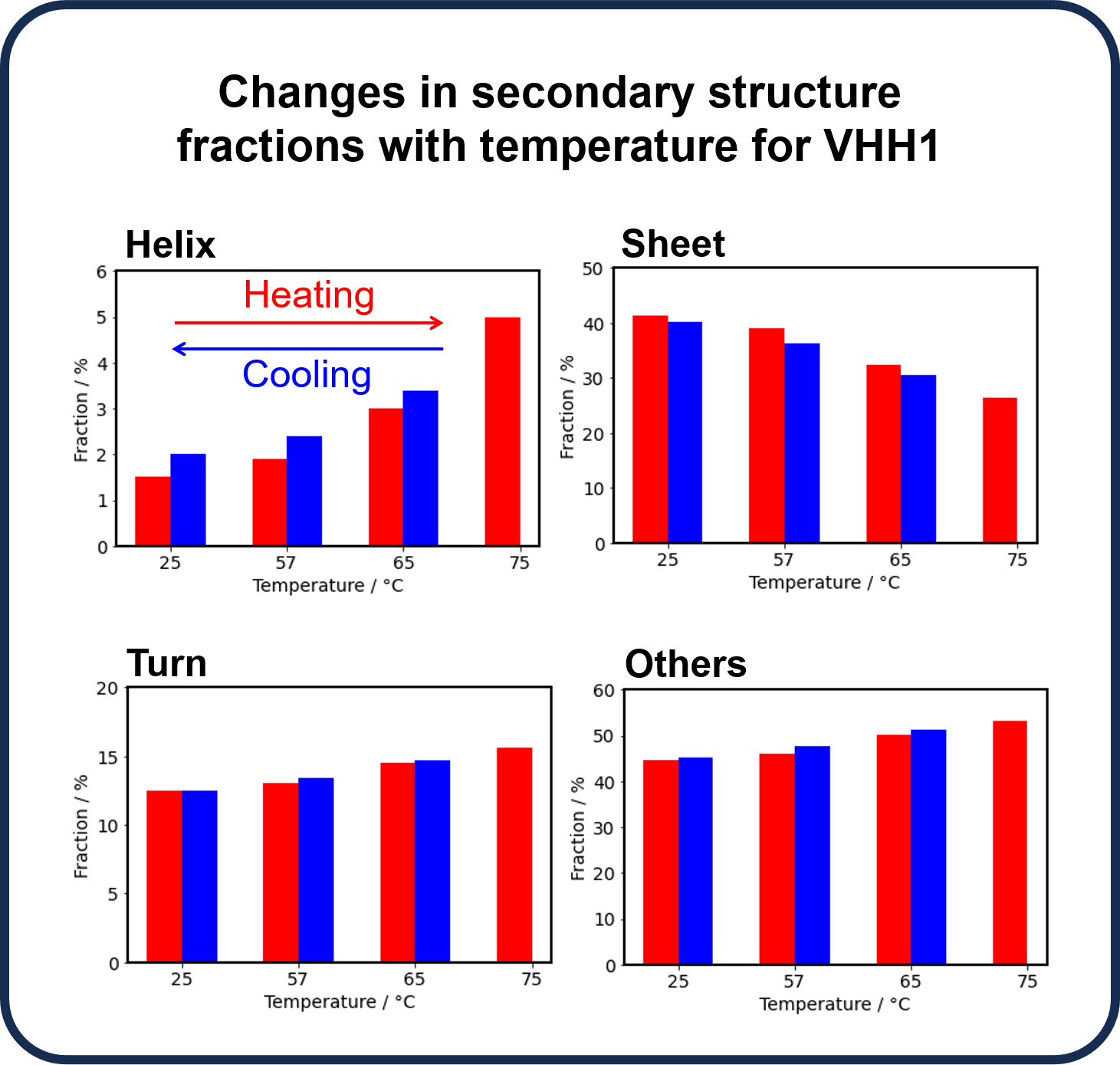
With increasing temperature, the fraction of β-sheets decreased whereas those of α-helix, turn, and other secondary structures increased due to thermal denaturation. Reversibility was observed for each secondary structure during the refolding process. Since the β-sheet structure forming the backbone of VHH is considered to have a significant effect on the structure of the CDR (binding site), we focused on β-sheets and determined the change before and after heating. The results showed that the refolding ability for VHH1 is 97.3%.
3.2 Dependence on pH and salt concentration
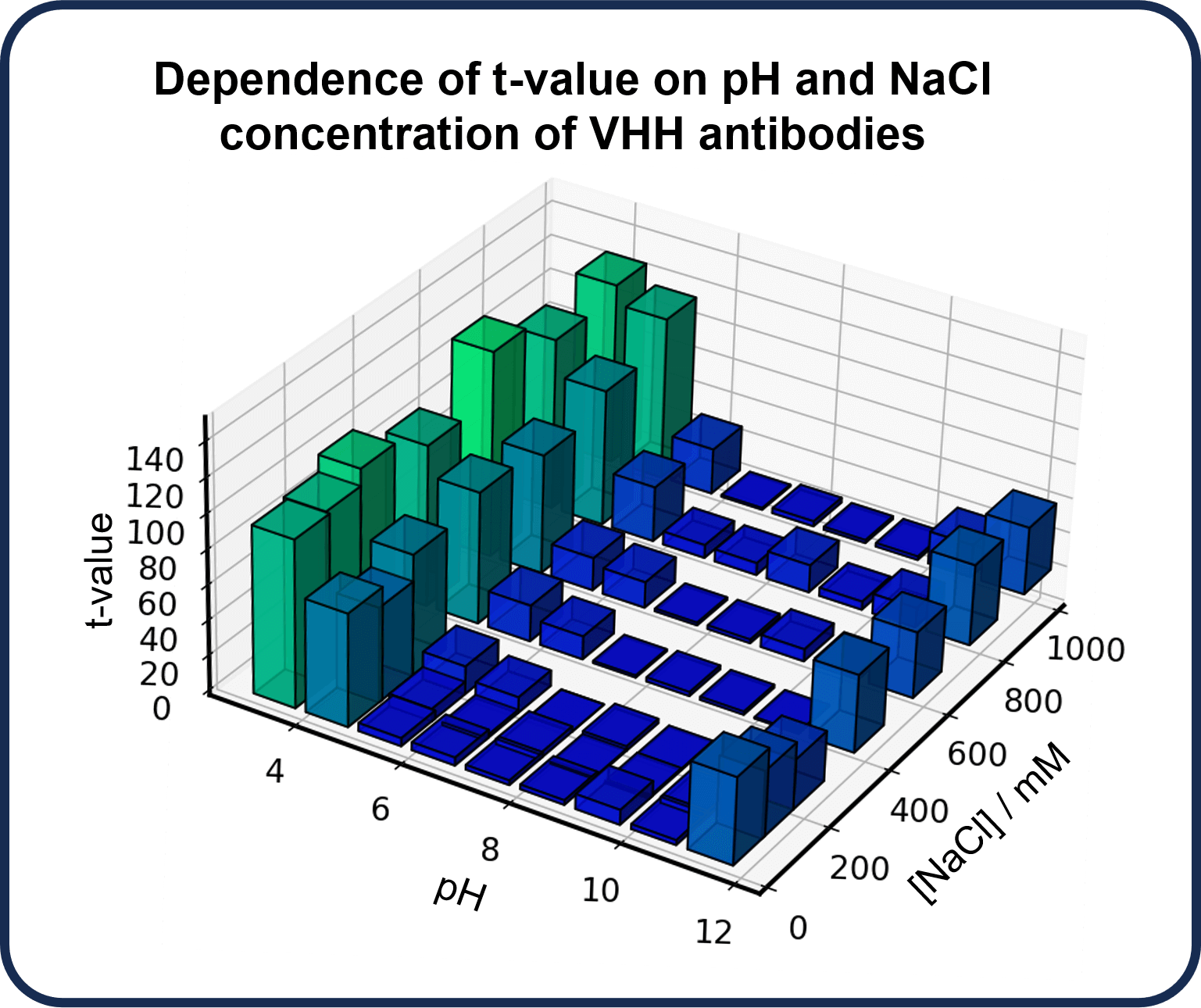
CD spectra of the anti-HAS VHH antibody, VHH4, under various pH conditions and salt concentrations were measured to examine the effect of solvent conditions. A VHH4 at pH 7 and NaCl concentration of 200 mM was used as a reference sample, and the change in the CD spectrum between the reference sample and samples under different conditions was quantified using t-values obtained from the qHOS program.
The results show that the t-values are small at pH 6-9, indicating that the structure is not significantly changed relative to the reference sample. On the other hand, below pH 5 or above pH 10, the t-values are larger, indicating that the structure changes significantly. Furthermore, the structure is also found to change with increasing NaCl concentration.
References
Poster Session at 15th Annual PEGS EUROPE (November 14 – 16, 2023, in LISBON, PORTUGAL)
Satoko Suzuki1, Emina Ikeuchi2, Taiji Oyama1, Ai Yamane1, Yasuo Horiguchi1, Ken-ichi Akao1, Akikazu Murakami3, András Micsonai4, József Kardos4, Koushi Nagamori1, and Kouhei Tsumoto2
1JASCO Corporation, Hachioji, Tokyo 192-8537, Japan
2Department of Chemistry & Biotechnology, School of Engineering, The University of Tokyo, Bunkyo-ku, Tokyo 113-8656, Japan
3RePHAGEN Co., Ltd., Uruma, Okinawa 904-2234, Japan
4ELTE NAP Neuroimmunology Research Group, Department of Biochemistry, Institute of Biology, ELTE Eötvös Loránd University, Budapest H-1117, Hungary
1) Proc. Natl. Acad. Sci. U.S.A., 112, E3095 (2015)
2) Nucleic Acids Res., 46, W315 (2018)
3) Methods in Molecular Biology (Clifton, N. J.), 2199, 175 (2021)
4) Front. Mol. Biosci., 9 (2022)
5) Nucleic Acids Res., 50, W90 (2022)
6) Appl. Spectrosc., 76 (2022)