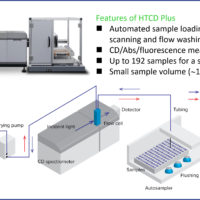
Introduction
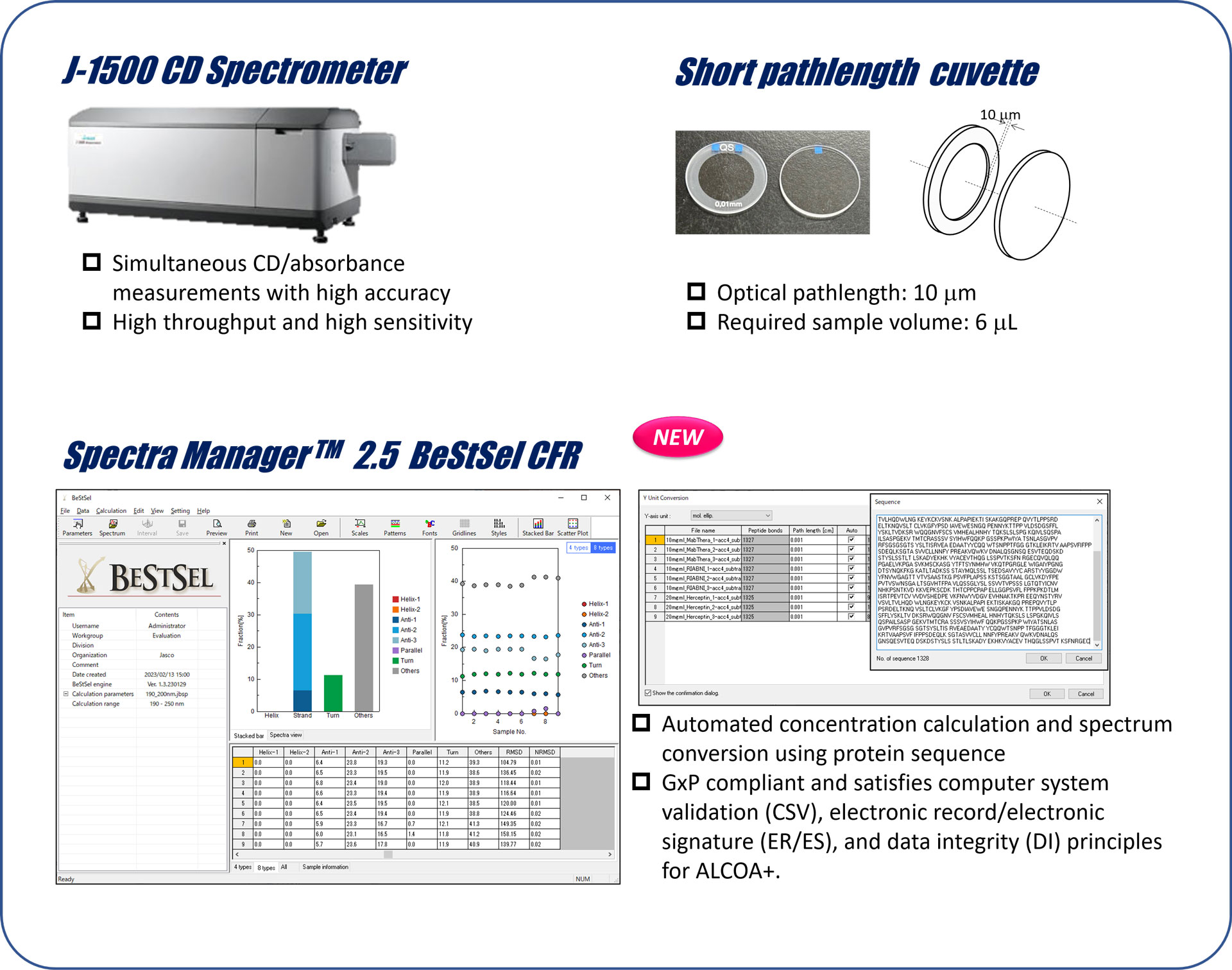
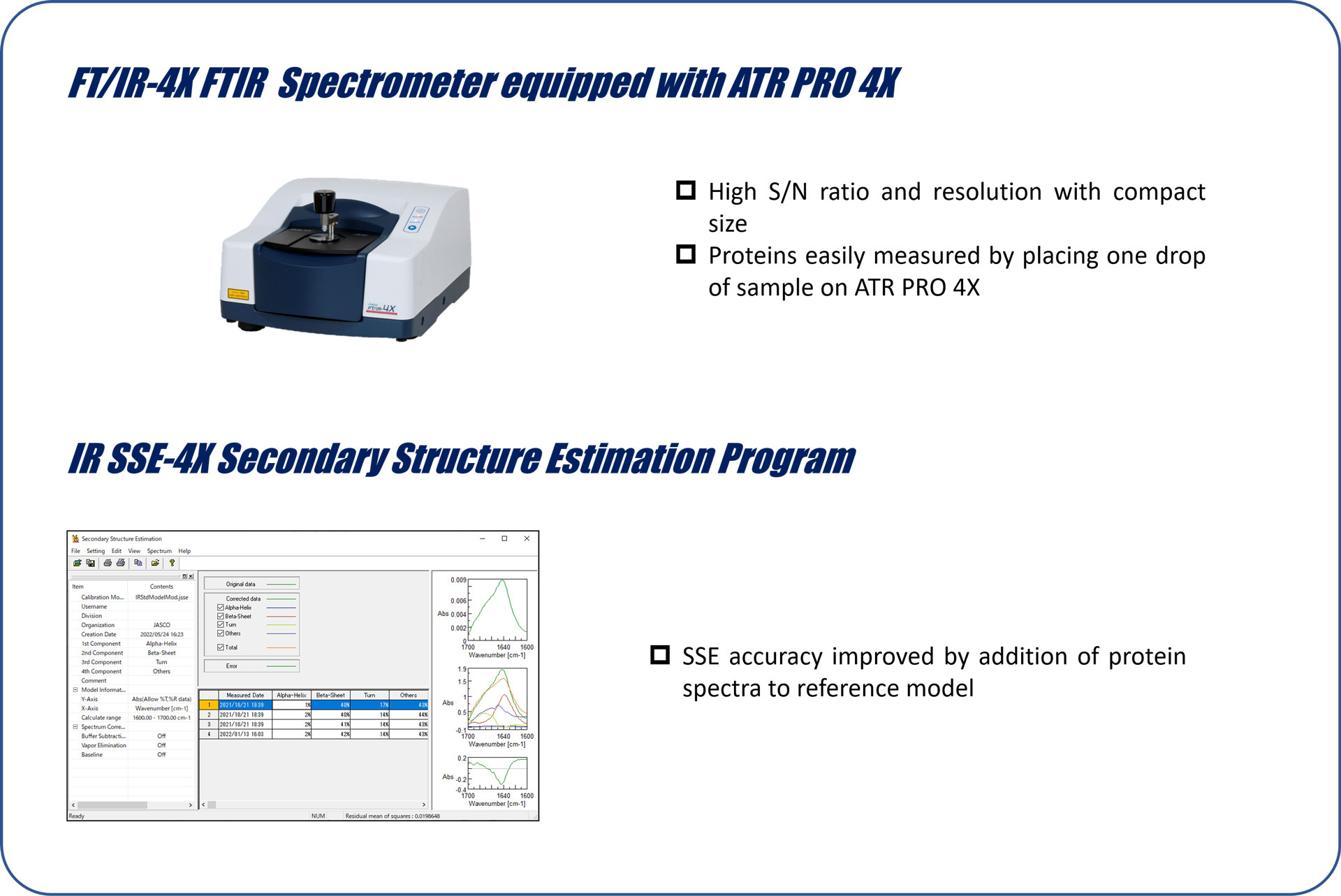
Higher-order structure (HOS) is an important antibody drug physicochemical property.1) The most well-known method for HOS characterization is circular dichroism (CD) spectroscopy. Although CD spectroscopy can provide robust structural information for low-concentration protein solutions (< ≈0.1 mg/mL), antibody drugs are formulated at concentrations higher than 10 mg/mL. Under these conditions, the antibody molecules exhibit physicochemical properties that differ from those under dilute conditions. Fortunately, evaluation of high concentration solutions can be done via short CD path lengths (~10 mm) or using FTIR with ATR. The ability to perform structural characterization of antibodies without dilution is extremely important because intermolecular interactions and changes in the HOS can lead to the formation of aggregates that cause serious immunogenicity.
Secondary structure estimation offers a route to evaluate protein higher order structure, but historically has not worked well on proteins with high amounts of β-sheet such as antibodies. Recently, Micsonai et al. developed the BeStSel algorithm that can accurately estimate the secondary structure from a CD spectrum by taking into account parallel-antiparallel orientations of β-strands and the twist of antiparallel β-sheets. BeStSel has the following features: 1) high estimation accuracy for a wide range of proteins, including β-strand-rich proteins such as antibodies, 2) provides eight types of secondary structure information, 3) capability to predict protein folding following the CATH classification, and 4) open web server.2-6)
While many academic researchers use the BeStSel web server, researchers in biopharma companies who need to work in a GxP environment have not been able to benefit from BeStSel. To make BeStSel accessible to biopharma, we developed Spectra Manager™ 2.5 BeStSel CFR as an add-in software for Spectra Manager™, a control and analysis platform for JASCO CD spectrometers, which is compatible with GxP.
Here, we describe an expanded application of CD spectroscopy for highly concentrated antibodies (undiluted) and report the results of a detailed secondary structure evaluation of antibody drugs using Spectra Manager™ 2.5 BeStSel CFR.
Experimental
Measurement system
Sample
- IgG, from rabbit serum (Sigma Aldrich): Powder dissolved in 20 mM citric acid buffer (150 mM NaCl, pH 6.0) from 5.8 to 23.1 mg/mL
- MabThera® (Rituximab, Roche) 10 mg/mL
- RIABNI™ (Rituximab, AMGEN) 10 mg/mL
- Herceptin® (trastuzumab, Roche): Powder dissolved in ultrapure water to 20 mg/mL
Measurement conditions
<CD measurement>
Bandwidth: 1.0 nm
Scan speed: 50 nm/min
D.I.T.: 4 sec
Accumulations: 4
<IR measurement>
Resolution: 4 cm-1
Detector: TGS
Accumulation: 128 times
N2 purge: On
Results
First, we examined the validity of measuring highly concentrated samples using short-pathlength cells with IgG from rabbit serum. Figs. 1a and 1b show simultaneously measured CD and absorption spectra for various concentrations of IgG. The intensity of the raw CD spectra increases linearly with concentration. The MRE spectra are obtained from raw CD spectra normalized by concentration. Concentration calculation and subsequent conversion to MRE were also automatically conducted by Spectra Manager™ 2.5 BeStSel CFR using a sequence of antibodies. The MRE spectra for each IgG concentration are consistent, and there is no change in the calculated secondary structure in this concentration range (Figs. 1c and 1e). The same samples were measured by FT-IR spectroscopy, which is an orthogonal method to CD, to verify the plausibility of the calculated secondary structure fractions (Fig. 1d). Secondary structure estimation (SSE) was performed for each concentration of antibody using the amide I peak at 1700-1600 cm-1. The secondary structure fractions calculated from the CD and FT-IR results show excellent agreement.
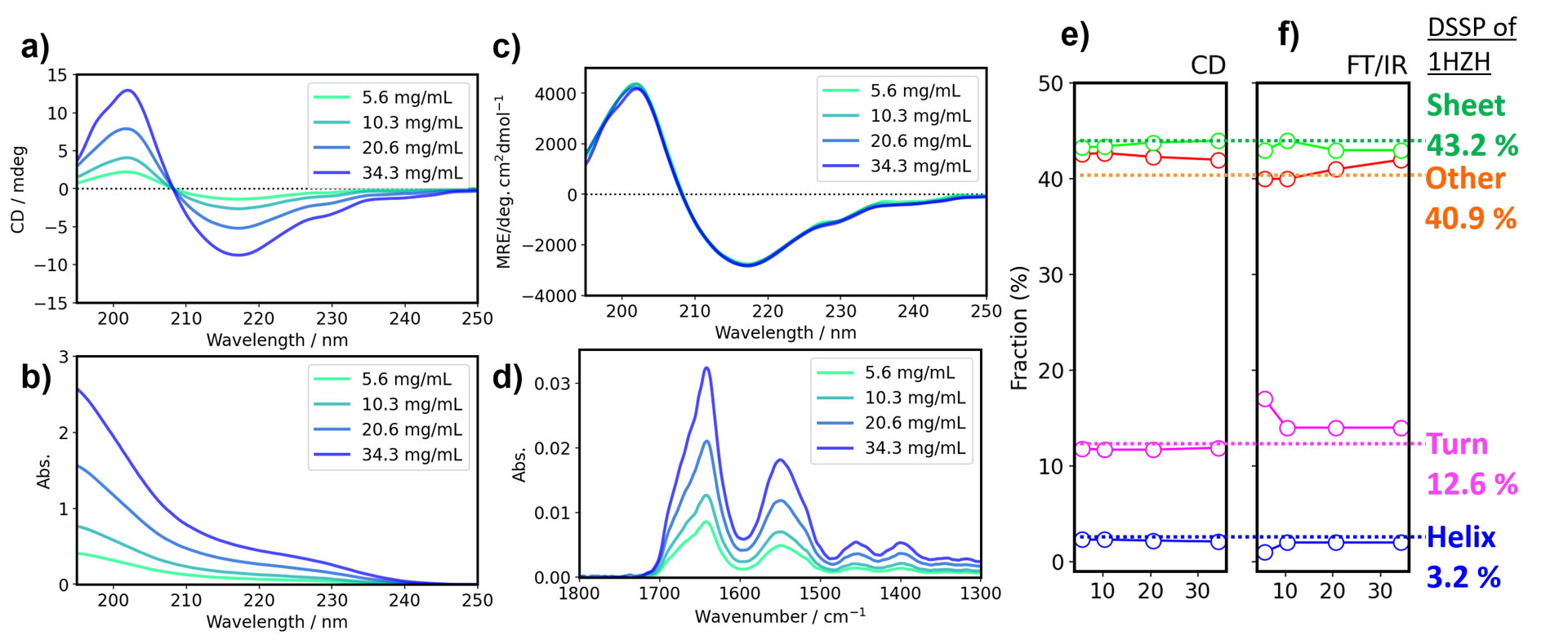
Figure 1. Comparison of secondary structure of antibodies at various concentrations using CD and FT-IR spectroscopy
a) CD spectra, b) absorbance spectra, c) MRE spectra in far-UV region, and d) IR spectra of rabbit serum IgG. e) and f) SSE results calculated using CD and FT-IR spectroscopy.
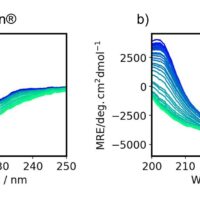
Having established a system to measure antibodies at high concentrations using a demountable cell, we then measured the actual antibody drug in its formulated (undiluted) form. The MRE spectra of each antibody drug are of very high quality even at short wavelengths (Fig. 2a). The standard deviation calculated from the results of three independent measurements is very small. The secondary structure fractions for MabThera® and RIABNI™ are consistent within the standard deviation of the three measurements (Fig. 2b). The experimental and recalculated fitted spectra show excellent agreement, and the normalized root-mean-square deviation (NRMSD), which indicates spectral differences, is also very small (Fig. 2a).
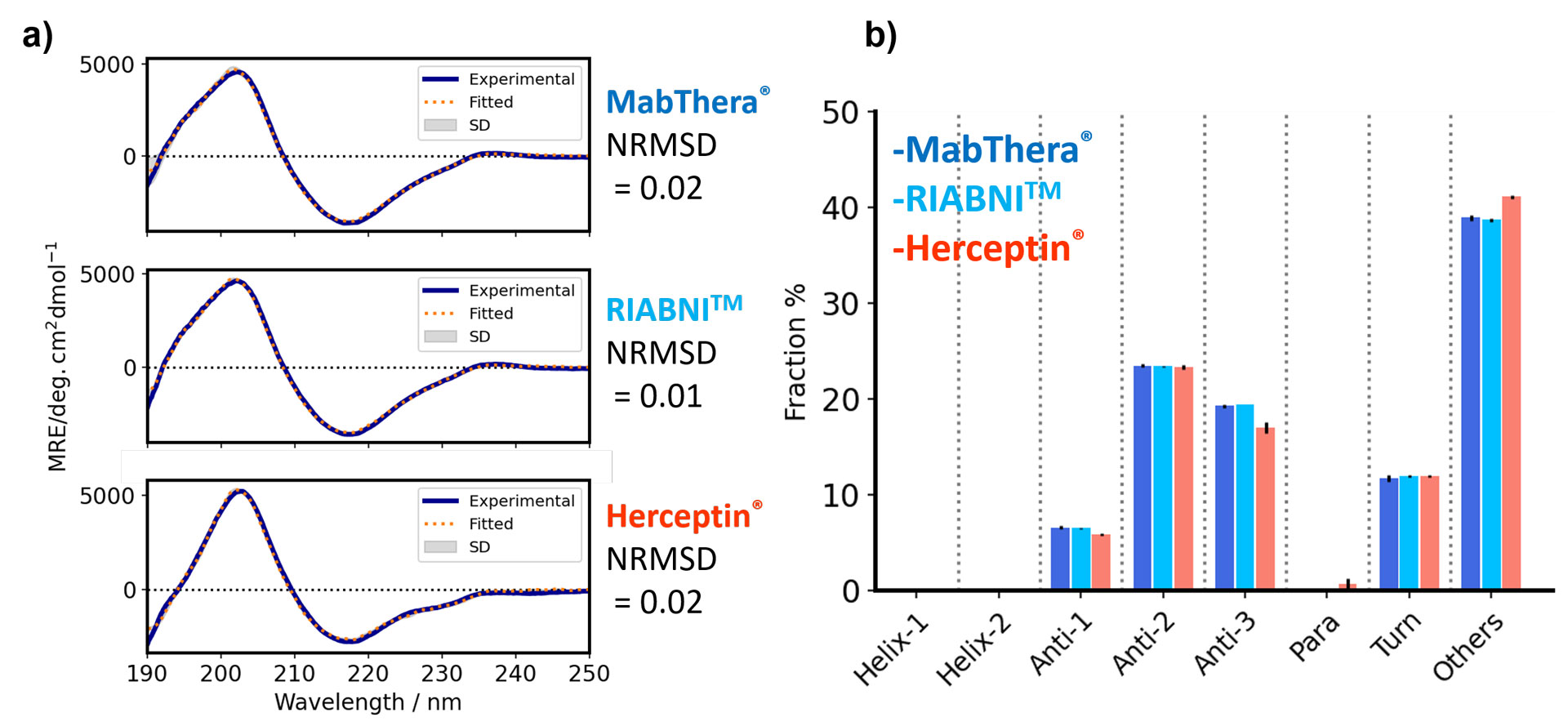
Figure 2. SSE results for antibody drugs using JASCO Spectra ManagerTM 2.5 BeStSel CFR
a) Mean of experimental spectra (solid line) and fitted spectra (dashed line) calculated using BeStSel are overlaid. The standard deviation was calculated from three independent measurements. b) Fraction of secondary structure for each antibody drug. Error bars indicate the standard deviation calculated from three independent measurements.
The combination of a short pathlength (10 µm) cell and Spectra Manager™ 2.5 BeStSel CFR with highly accurate absorbance data measured simultaneously by CD spectroscopy allowed us to evaluate the HOS of the antibody in its formulated state without dilution.
References
Poster Session at 20th Annual PEGS Boston Summit (May 15 – 19, 2023, in BOSTON, MA, U.S.A.)
Satoko Suzuki1, Forrest R. Kohl2, Taiji Oyama1, Ai Yamane1, András Micsonai3, József Kardos3, Ken-ichi Akao1
1JASCO Corporation, Hachioji, Tokyo 192-8537, Japan,
2JASCO Inc., Easton, MD, USA,
3ELTE NAP Neuroimmunology Research Group, Department of Biochemistry, Institute of Biology, ELTE Eötvös Loránd University, Budapest H-1117, Hungary
1) Ama, D., Hasegawa, J., Uchiyama, S., and Fukui, K. (2010). Netsu sokutei (Japan), 38, 9-15.
2) Micsonai, A., Wien, F., Kernya, L., Lee, Y. H., Goto, Y., Réfrégiers, M., & Kardos, J. (2015). Proc. Natl. Acad. Sci. U.S.A., 112, E3095–E3103.
3) Micsonai, A., Wien, F., Bulyáki, É., Kun, J., Moussong, É., Lee, Y. H., Goto, Y., Réfrégiers, M., & Kardos, J. (2018). Nucleic Acids Res., 46, W315-W322.
4) Micsonai, A., Bulyáki, É., & Kardos, J. (2021). Methods in molecular biology (Clifton, N.J.), 2199, 175–189.
5) Micsonai, A., Moussong, É., Lee, Y. H., Murvai, N., Tantos, Á., Tőke, O., Réfrégiers, M., Wien, F., & Kardos, J. (2022). Front. Mol. Biosci., 9.
6) Micsonai, A., Moussong, É., Wien, F., Boros, E., Vadászi, H., Murvai, N., Lee, Y. H., Molnár, T., Réfrégiers, M., Goto, Y., Tantos, Á., & Kardos, J. (2022). Nucleic Acids Res., 50, W90-W98.