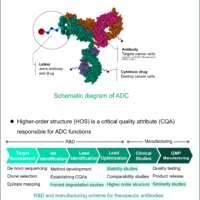
The market for therapeutic antibodies has been dramatically expanding over the past decades. Antibody drugs exhibit therapeutic effects, such as inhibiting the growth of malignant tumor cells and the activity of immune cells by binding antigens expressed in target cells with high affinity and specificity. Such antibody drugs are expected to be effective therapeutic agents for unmet medical needs.
The higher order structures (HOS) formed by therapeutic antibodies determines their efficacy. Because antibodies are unstable macromolecules, their HOS can be affected by various stimuli during the manufacturing process, resulting in reduced drug efficacy and impurities derived from the target substance. Therefore, HOS is considered to be a Critical Quality Attribute (CQA) and is subject to similarity studies, stability studies, and forced degradation studies in the R&D process from target assessment to lead optimization. In addition, it is monitored during the post-launch manufacturing process.
Antibody structures are hierarchically formed as primary, secondary, tertiary, and quaternary structures, and it is important to comprehensively evaluate each structure in the characterization of antibodies. This application eBook presents examples of evaluations using various analytical instruments, including Circular Dichrosim (CD) spectrometers, Fourier-transform infrared (FTIR) spectrometers, Raman imaging microscopes and High Performance Liquid Chromatography (HPLC).
CONTENTS
Structure Studies
Secondary structure
Secondary structure prediction for Herceptin® using CD spectroscopy
Secondary structure prediction for IgG using IR spectroscopy
Orthogonal assessment of secondary structure for IgG
Tertiary structure
Near-UV CD measurement of high-concentration IgG
Quaternary structure
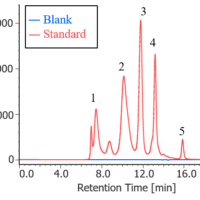
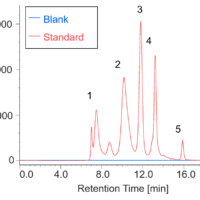
Assessment of aggregates in antibody drugs by SEC
Similarity Studies
Single assessment
Orthogonal tertiary structure similarity assessment using near-UV CD and fluorescence spectroscopy
Orthogonal assessment
4-way Orthogonal HOS similarity assessment of biosimilars using multiple spectroscopic techniques and statistical calculations
Stability Studies
Thermal denaturation
Thermal denaturation analysis of monoclonal antibodies using CD spectroscopy
Thermal denaturation analysis of monoclonal antibodies using FTIR spectroscopy
Thermal denaturation analysis of monoclonal antibodies using Raman spectroscopy
Heat-induced denaturation and refolding study of VHH antibodies
pH & salt denaturation
pH & salt denaturation analysis of VHH antibodies using CD spectroscopy
Forced Degradation Studies
]Monitoring of aggregates and degradation products by SEC in FDS of antibody drugs
Statistical similarity assessment of therapeutic antibodies in FDS using CD spectroscopy