Supercritical fluid extraction (SFE) is a method in which supercritical fluid as an extraction medium is added to substances containing target components, and extraction is performed based on differences in solubility. In particular, the use of supercritical carbon dioxide as an extraction medium offers many advantages in a variety of fields, including short extraction times, simplified operation, and improved extraction efficiency compared with the organic solvent extraction method. Also, solvent removal following extraction is much easier, allowing more accurate component concentrations to be determined.
Because the critical temperature of carbon dioxide is only 31 °C, extraction is possible close to room temperature. Furthermore, extraction can be carried out in a carbon dioxide atmosphere without oxygen. This means that this method can be used for substances that are temperature-sensitive or easily oxidized. Supercritical carbon dioxide is effective at dissolving lipid-soluble or low-polar compounds, but it can be adapted to the extraction of highly polar components by adding alcohols such as methanol or organic solvents such as acetonitrile and tetrahydrofuran as auxiliary solvents (referred to as entrainers).
In recent years, it has attracted attention as an environmentally friendly extraction method that does not use harmful organic solvents, in line with the concept of Green Chemistry.
Table 3 Comparison of conventional organic solvent and supercritical carbon dioxide extraction and pretreatment methods
| Organic solvent | Disadvantages |
| Soxhlet extraction & purification | Uses harmful organic solvents |
| Solvent extraction & purification | Process is complicated and time-consuming Long pre-processing time Higher sample concentration required Trace residual solvent |
| Supercritical carbon dioxide | Advantages |
| Carbon dioxide | Uses safe carbon dioxide |
| Carbon dioxide & ethanol, etc. | Simple process that can be automated Fast pre-processing and extraction Selective extraction possible Online SFE-SFC Prevention of sample oxidation, operation at low temperature |
Table 4 Applications of supercritical carbon dioxide extraction
| Field | Material | Purpose |
| Food | Coffee beans | Decaffeinated |
| Tea | Flavor ingredient | |
| Hop | Hop extract | |
| Red pepper | Spicy extract, pigment | |
| Fish meal | Fish oil | |
| Fish oil | EPA, DHA | |
| Plant | Essential oils, pigments, medicinal ingredients | |
| Vegetable oil | Vitamin E | |
| Pharmaceutical | Natural products | Medicinal ingredients, bioactive substances |
| Tablet | Residual solvent | |
| Chemical | Coal, oil, energy Plastic, rubber, polymer Metal Inorganic compounds and ceramics Aerogel Electrical / electronic parts |
Low boiling point component |
| Polymerization solvent | ||
| Monomer | ||
| Oligomer | ||
| Impurities | ||
| Nasty smell | ||
| Developing agent | ||
| Degreasing | ||
| Dehydration | ||
| Solvent removal | ||
| Washing | ||
| Others | Cigarettes | Nicotine |
| Soil | Organic matter |
Supercritical carbon dioxide extraction system
The basic configuration and flow diagram of the SFE system are shown in Fig. 6. The system is configured with a liquefied carbon dioxide feed pump (2), an entrainer feed pump (5), a high-pressure vessel (8), a thermostatic bath (10), an optional detector for monitoring the extraction process, and a back-pressure regulator valve for controlling the extraction pressure (11).
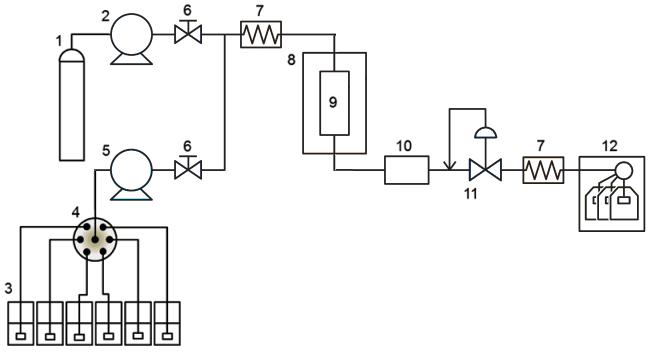
Fig. 6 Configuration of supercritical fluid extraction system
For the high-pressure-resistance vessel, a column-type or cup-type design can be selected according to the state of the extracted sample, and various capacities are available. The extraction conditions can be set by changing the pressure, temperature, and modifier solvent type and amount as parameters. It is also possible to attach a viewing cell that allows real-time observation of sample extraction and reaction processes under supercritical conditions from the observation window.
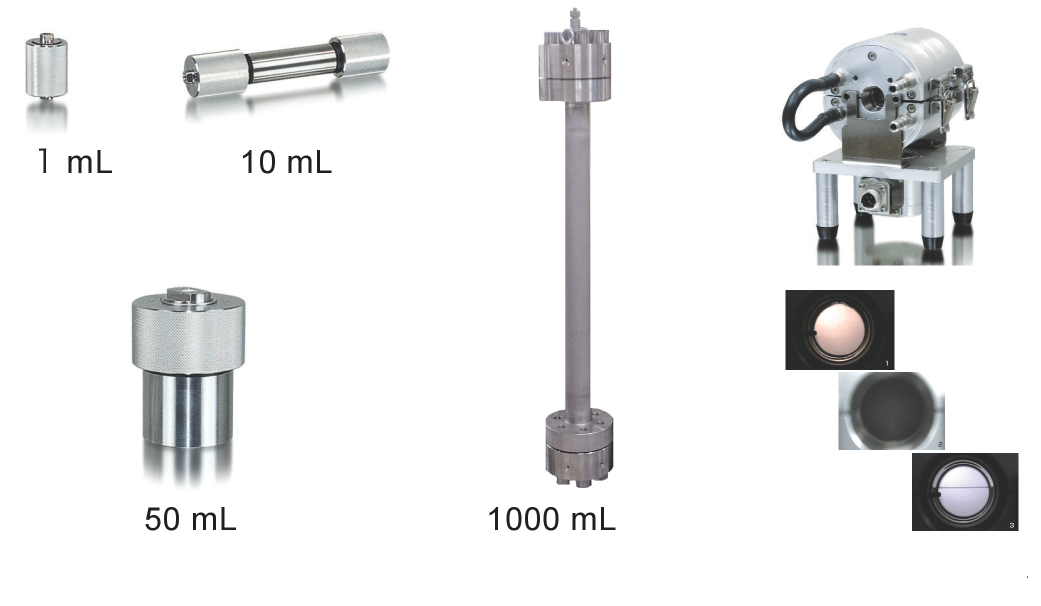
Fig. 7 Pressure vessels used in SFE system (Upper left: column type, Lower left: glass type, Center: tower type, Right: observation type)