Introduction
The pre-column derivatization method for amino acid analysis is a technique where amino acid samples are first derivatized and then separated using a column, such as a C18 column and detected. One of the derivatization reagents known for reacting with primary amino acids is o-phthalaldehyde (OPA). However it does not react with secondary amino acids like proline. On the other hand, proline is also important amino acids that constitutes proteins and that is found in many foods and beverages. Therefore, there are situations where it becomes necessary to analyze amino acids, including proline. To address this, the pre-column derivatization method using both OPA and 9-fluorenylmethyl chloroformate (FMOC) can be employed. In this method, primary amino acids are reacted with OPA, while secondary amino acids that do not react with OPA are derivatized with FMOC. Subsequently, each amino acid is detected as a fluorescent derivative.
Although these fluorescent derivatives have different excitation and emission wavelengths, simultaneous analysis can be achieved by automatically switching the detection wavelength. Furthermore, the User Program of the autosampler allows for the automation of the derivatization process, including dispensing, mixing, and agitation of each reaction solution.
In this study, we report the measurement of samples containing amino acids using the pre-column derivatization method with OPA and FMOC under RHPLC conditions.
LC-4000 RHPLC system
Experimental
Instruments
Pump: PU-4185-Binary
Autosampler: AS-4150*
Column oven: CO-4060
Detector: FP-4020*
* with option units
Conditions
Column: Unifinepak C18 (3.0 mmI.D. x 75 mmL, 1.9 µm)
Eluent A: 20 mM phosphate potassium buffer (adjusted to pH 6.9)
Eluent B: Acetonitrile/methanol/water (45/40/15)
Gradient: A/B = 89/11 (0.0 min) 87/13 (3.0 min) →69/31 (5.0 min) →63/37 (7.0 min) → 30/70 (11.0 min) → 0/100 (11.5 min) → 0/100 (14.5 min) →89/11 (15.0 min), 1cycle; 20 min
Flow rate: 0.8 mL/min
Reagent: 3-mercaptopropionic acid solution (10 µL in 0.1 mol/L borate buffer (pH 9.2) 10 mL), OPA solution (20 mg in 0.1 mol/L borate buffer (pH 9.2) 10 mL), FMOC solution (2 mg in acetonitrile 10 mL)
Column temp.: 35 ºC
Wavelength: Ex. 340 nm, Em. 450 nm → Ex. 266 nm, Em. 305 nm (10.5 min) (Gain x10)
Injection volume: 1 µL
Standard: 22 amino acids each in 0.1 M hydrochloric acid (Refer to each data for concentrations.)
Reaction formula

Keywords
RHPLC, Fluorescence Detection, Pre-column Derivatization, OPA, FMOC, Amino Acids, Unifinepak C18
Results
Measurement result of system’s performance verification
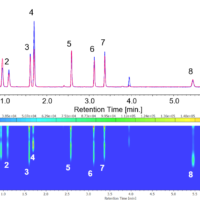
To verify the system’s performance, we conducted measurements of an amino acid standard solution. Figure 1 shows the chromatogram of a 100 µmol/L amino acid standard solution, and Table 1 demonstrates the reproducibility of retention times and peak areas for each component. We have confirmed excellent reproducibility for all peaks, with retention times being less than 0.2 % and peak areas less than 3 %.
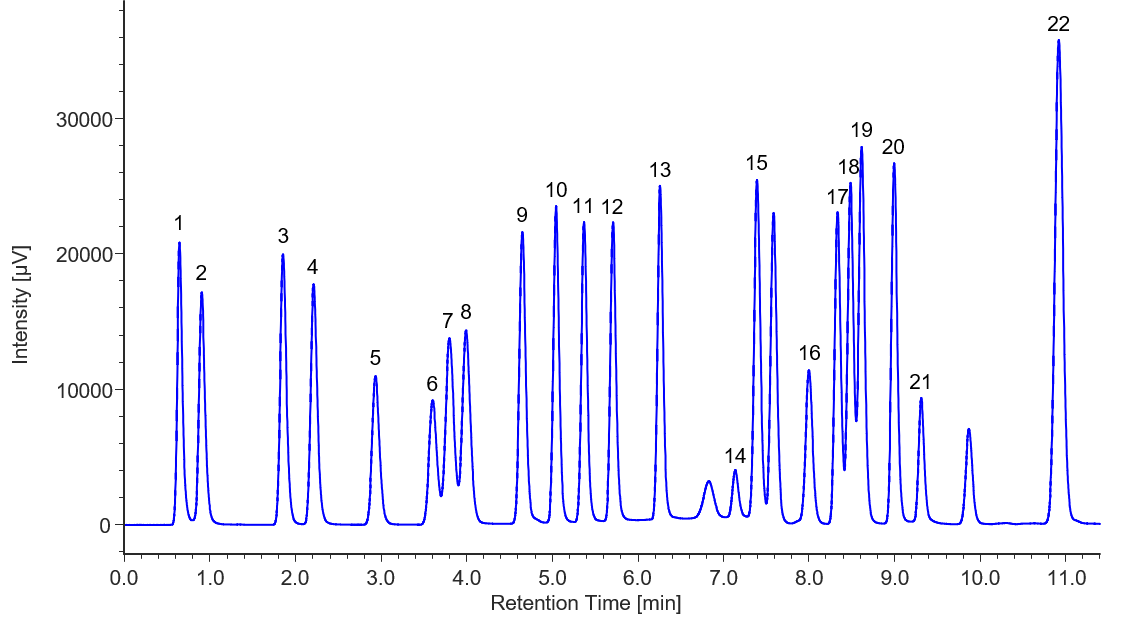
Fig. 1 Chromatogram of the amino acid standard solution (100 µmol/L)
1: Aspartic acid, 2: Glutamic acid, 3: Asparagine, 4: Serine, 5: Glutamine, 6: Histidine, 7: Glycine, 8: Threonine, 9: Citrulline, 10: Arginine, 11: Alanine, 12: GABA, 13: Tyrosine, 14: Cystine, 15: Valine, 16: Methionine, 17: Tryptophan, 18: Phenylalanine, 19: Isoleucine, 20: Leucine, 21: Lysine, 22: Proline
Table 1 Reproducibility and detection limit for the standard solution (100 µmol/L, n = 6)
| Peak | RSD [%] | Detection limit [fmol] * (S/N = 3) | Peak | RSD [%] | Detection limit [fmol] * (S/N = 3) | ||
| Retention time | Peak area | Retention time | Peak area | ||||
| Aspartic acid | 0.00 | 0.23 | 130 | GABA | 0.02 | 0.59 | 124 |
| Glutamic acid | 0.15 | 0.12 | 158 | Tyrosine | 0.02 | 0.27 | 110 |
| Asparagine | 0.17 | 0.38 | 136 | Cystine | 0.07 | 0.78 | 775 |
| Serine | 0.15 | 0.32 | 153 | Valine | 0.06 | 0.51 | 108 |
| Glutamine | 0.16 | 0.64 | 245 | Methionine | 0.15 | 0.80 | 243 |
| Histidine | 0.15 | 0.79 | 298 | Tryptophan | 0.15 | 0.39 | 118 |
| Glycine | 0.13 | 1.04 | 200 | Phenylalanine | 0.15 | 0.30 | 108 |
| Threonine | 0.12 | 0.34 | 189 | Isoleucine | 0.15 | 0.25 | 97 |
| Citrulline | 0.08 | 0.30 | 126 | Leucine | 0.15 | 0.30 | 102 |
| Arginine | 0.06 | 0.31 | 116 | Lysine | 0.17 | 0.62 | 297 |
| Alanine | 0.03 | 0.28 | 122 | Proline | 0.12 | 2.06 | 78 |
* Calculated from the measurement for standard solution at 100 µmol/L.
Measurement result of linearity of calibration curve
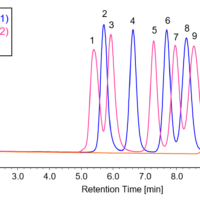
Figure 2 presents the results of measurements conducted with a 6-point standard solution in the concentration range of 1.0 to 100 µmol/L. The correlation coefficients for the calibration curves were all greater than or equal to r = 0.9998, indicating excellent linearity.
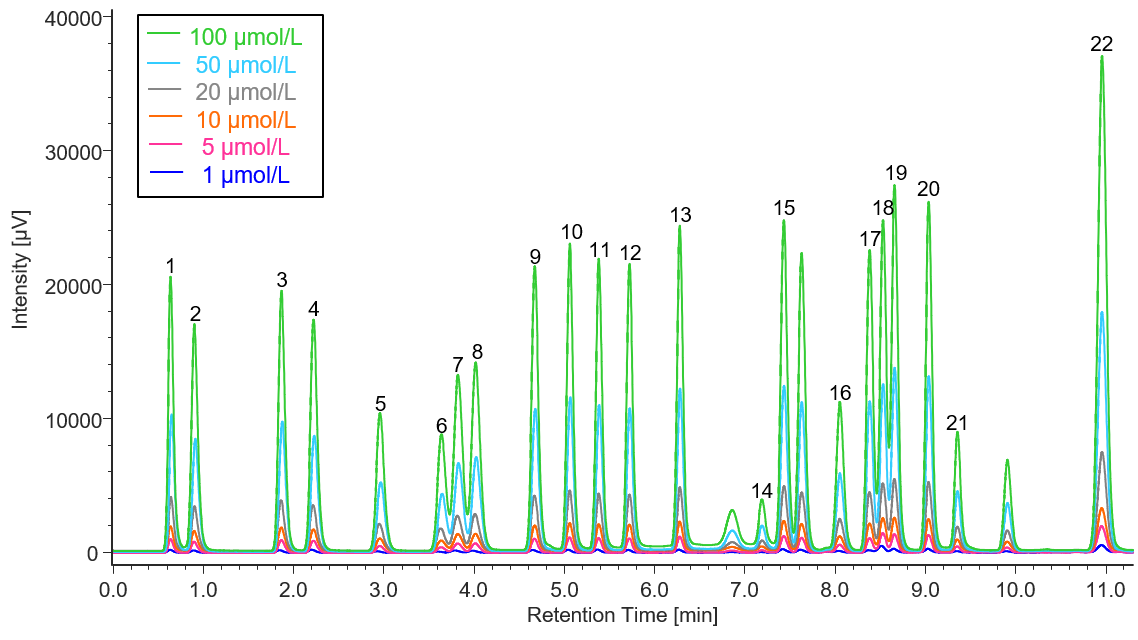
Fig. 2 Chromatogram of the amino acid standard solution (1, 5, 10, 20, 50, 100 µmol/L)
1: Aspartic acid, 2: Glutamic acid, 3: Asparagine, 4: Serine, 5: Glutamine, 6: Histidine, 7: Glycine, 8: Threonine, 9: Citrulline, 10: Arginine, 11: Alanine, 12: GABA, 13: Tyrosine, 14: Cystine, 15: Valine, 16: Methionine, 17: Tryptophan, 18: Phenylalanine, 19: Isoleucine, 20: Leucine, 21: Lysine, 22: Proline
Table 2 Correlation coefficients for the calibration curves of the standard samples
| Peak | Correlation coefficients | Peak | Correlation coefficients |
| Aspartic acid | 1.0000 | GABA | 1.0000 |
| Glutamic acid | 1.0000 | Tyrosine | 1.0000 |
| Asparagine | 0.9999 | Cystine | 0.9999 |
| Serine | 1.0000 | Valine | 1.0000 |
| Glutamine | 0.9999 | Methionine | 0.9999 |
| Histidine | 1.0000 | Tryptophan | 1.0000 |
| Glycine | 1.0000 | Phenylalanine | 0.9999 |
| Threonine | 1.0000 | Isoleucine | 1.0000 |
| Citrulline | 0.9999 | Leucine | 1.0000 |
| Arginine | 0.9999 | Lysine | 1.0000 |
| Alanine | 1.0000 | Proline | 0.9999 |
Measurement result of actual samples
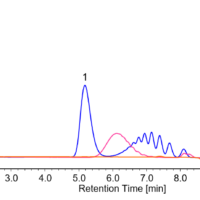
Figures 3 and 4 show chromatograms of soy sauce and sports drink.
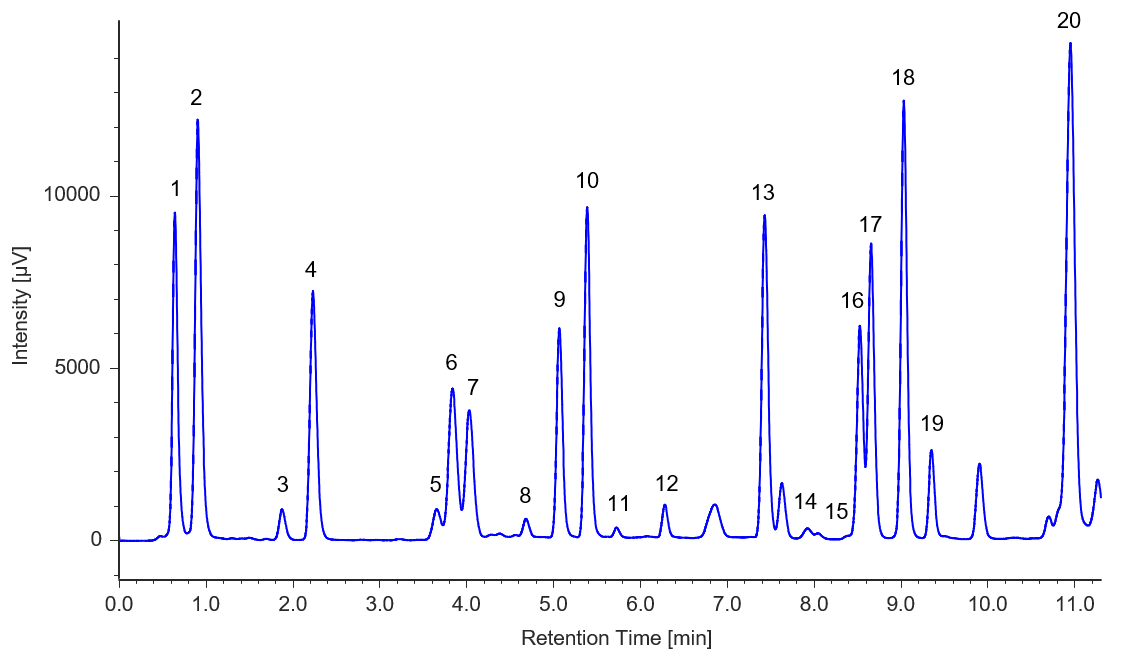
Fig. 3 Chromatogram of soy sauce
1: Aspartic acid, 2: Glutamic acid, 3: Asparagine, 4: Serine, 5: Histidine, 6: Glycine, 7: Threonine, 8: Citrulline, 9: Arginine, 10: Alanine, 11: GABA, 12: Tyrosine, 13: Valine, 14: Methionine, 15: Tryptophan, 16: Phenylalanine, 17: Isoleucine, 18: Leucine, 19: Lysine, 20: Proline
Sample Preparation Methods:
1) Dilution by a factor of 1000 with 0.1 M HCl
2) Filtration through a 0.45 µm membrane filter
3) Filtration through a 0.2 µm membrane filter
4) Injection into the HPLC system
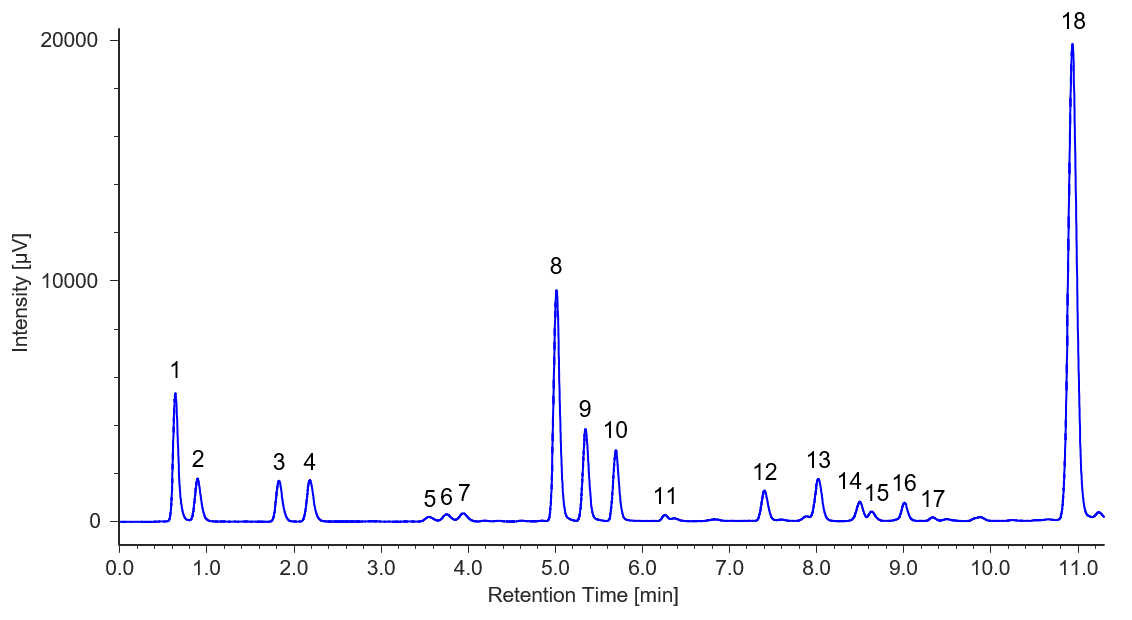
Fig. 4 Chromatogram of sports drink
1: Aspartic acid, 2: Glutamic acid, 3: Asparagine, 4: Serine, 5: Histidine, 6: Glycine, 7: Threonine, 8: Arginine, 9: Alanine, 10: GABA, 11: Tyrosine, 12: Valine, 13: Methionine, 14: Phenylalanine, 15: Isoleucine, 16: Leucine, 17: Lysine, 18: Proline
Sample Preparation Methods:
1) Dilution by a factor of 2 with 0.1 M HCl
2) Filtration through a 0.45 µm membrane filter
3) Filtration through a 0.2 µm membrane filter
4) Injection into the HPLC system
Automatic pre-column derivatization using the autosampler
Figure 5 shows the steps for automatic pre-column derivatization using the User Program of the AS-4150 autosampler used in this study. By utilizing this program, it becomes possible to perform derivatization automatically with just the preparation of samples, reagent solutions, and mixing vials.
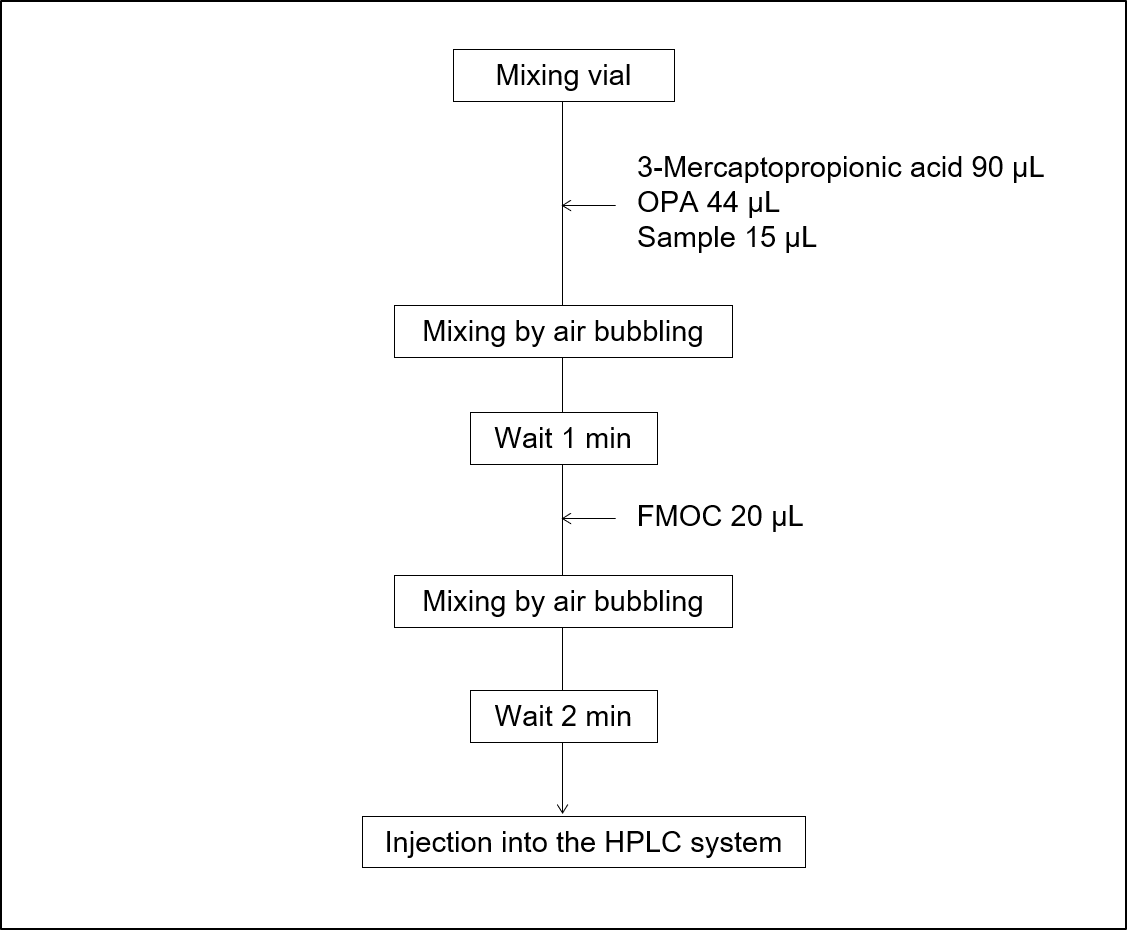
Fig. 5 Procedures for pre-column derivatization with the autosampler
Conclusion
Through this experiment, it was confirmed that the measurement of samples containing amino acids using the pre-column derivatization method with OPA and FMOC under RHPLC conditions can successfully separate and detect amino acids including proline.