RePHAGEN has created the S1 sub-unit of spike protein from the base sequence of Coronavirus (SARS-CoV-2) from public database Genbank as antigen, and by using their own VHH antibody (variable domain of heavy chain of heavy chain antibody) library, they have discovered the VHH antibody which has high specificity against the receptor binding domain of SARS-CoV-2. In addition, they have created VHH antibody with high binding ability by shuffling one part of CDR (complementarity determining region). In order to estimate its secondary structure and to evaluate the thermal stability and refolding ability, we have measured 3 kinds of VHH antibody samples which RePHAGEN provided by using J-1500 CD spectrometer and FT/IR-6600 FTIR spectrometer.
Stability evaluation of anti-Coronavirus VHH antibody
Since the structure of antibody drugs may change and cause a lose in their activity depending on the formulation conditions and storage environment, it is necessary to evaluate their stability in order to ensure quality. In particular, thermal denaturation is highly reversible depending on the protein, increasing the need to determine denaturation temperature and refolding ability in the stability evaluation of antibody drugs.
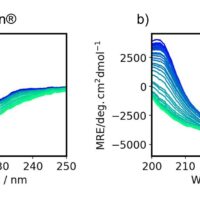
Here, we evaluated the thermal stability and refolding ability of 3 different types of VHH antibodies using a J-1500 CD spectrometer. The secondary structure percentages were estimated using the BeStSel program [Beta Structure Selection, provided by Department of Biochemistry Institute of Biology Eötvös Loránd University (HUNGARY)] to further correlate the denaturation temperatures with structural changes to VHH antibody framework.
Analysis of the effects of low temperature on the secondary structure of VHH antibodies
Antibody based drugs are typically stored in a refrigerated or frozen state, but this may lead to denaturation from changes in temperature or repeated freezing and thawing. Therefore, it is essential to evaluate the structural stability in order to maintain quality control.
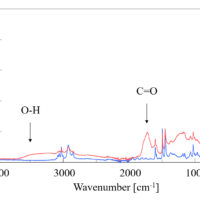
FTIR spectroscopy has been widely used to evaluate the structure of proteins such as VHH antibodies. Secondary structure estimation of α-Helices and β-Sheets by FTIR spectroscopy can be performed quickly and easily. Here, we discussed the results of FTIR measurements of secondary structure changes caused by the cooling process and repeated freezing and thawing of a VHH antibody that binds to SARS-CoV-2.

Evaluation of HOS identity about VHH antibody binding to novel coronavirus
The structure of biopharmaceuticals is sensitive to changes in the manufacturing process or storage environment and it is necessary to evaluate the HOS (higher order structure) of these molecules sustained after any processing or storage alterations.

Here, we evaluated the HOS of three types of anti-SARS-CoV-2 VHH antibodies using the [Spectrum QC Compare Test] program that quantitatively determines the significance in structure changes based on the difference in CD spectral shape.

*1 RePHAGEN Co., Ltd.
RePHAGEN is generating antibodies from VHH library with 20 billion diversity, and is developing fungicides and resistant bacterial infections using natural phage.
Okinawa Life Science Research Center Room 120, 5-8 Suzaki, Uruma City, Okinawa, JAPAN (postal code 904-2234)

*2 BeStSel
Micsonai. A, BeStSel: a web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra, , Nucleic Acids Res, 46 (2018), 315-322 (Link to Nucleic Acids Research)
Micsonai. A, Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy, PNAS, 112 (2015), 3095-3103 (Link to PNAS)