Introduction
Antibody pharmaceuticals have attracted a great deal of attention in recent years since they can efficiently target specific disease-causing molecules and they are associated with few side effects. However, unlikely conventional low-molecular-weight drugs, biopharmaceuticals can easily lose their activity due to high-order structural changes that depend on conditions such as temperature, pH and salt concentration. It is therefore important to investigate drug stability under various conditions during the development, formulation and quality control of antibody drugs. In particular, it is known that denaturation due to temperature can be reversible depending on the protein involved, so evaluation of reversibility is important for assessing the stability of antibody drugs.
CD and fluorescence measurements can easily provide information on the secondary and tertiary structure of proteins at low concentrations under homogeneous conditions. In the present study, we carried out reversibility measurements on VHH antibodies, which have become a topic of great recent interest, using simultaneous CD/fluorescence measurements. We describe a method for quantifying spectral change points with higher accuracy based on Euclidean distance.
Experimental
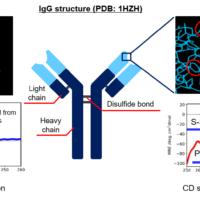
VHH Antibody
Camel heavy-chain antibody
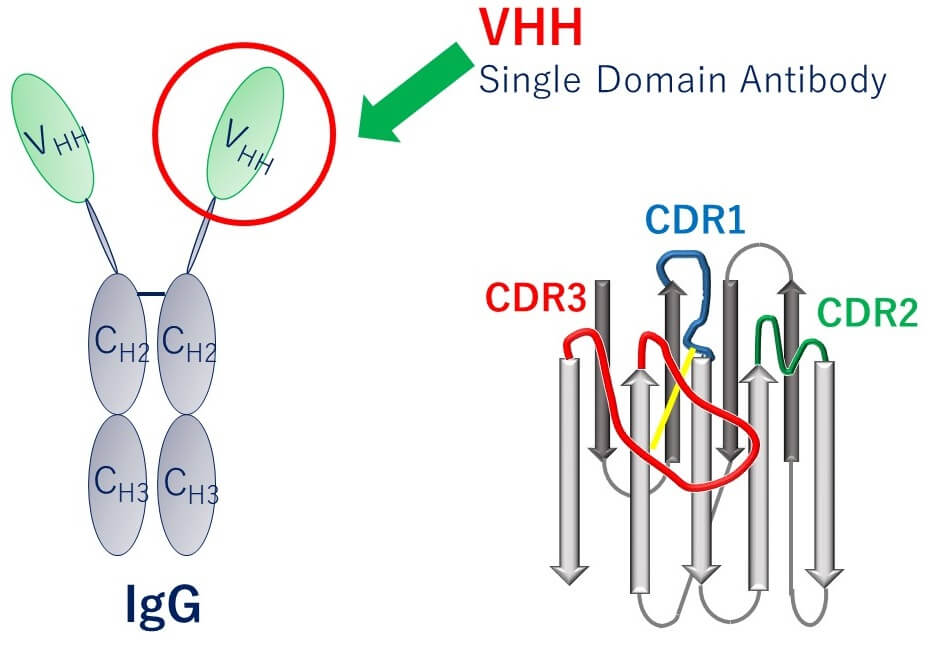
- High stability (temp., pH, modifier)
- Mass production in E.coli
- Affinity same as normal antibodies
- Easy to modify
Measurement
Measurement conditions
- Sample concentration: 0.0488 mg/mL (PBS, pH7.4)
- Cell: 10 mm
- Temp. slope: 1 degree C/min

- Simultaneous measurement of CD and fluorescence
- Easy operation without calibration or alignment
- 6-position measurements using a turret cell changer
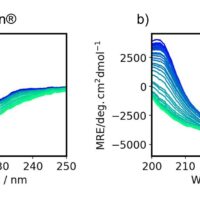
Spectra
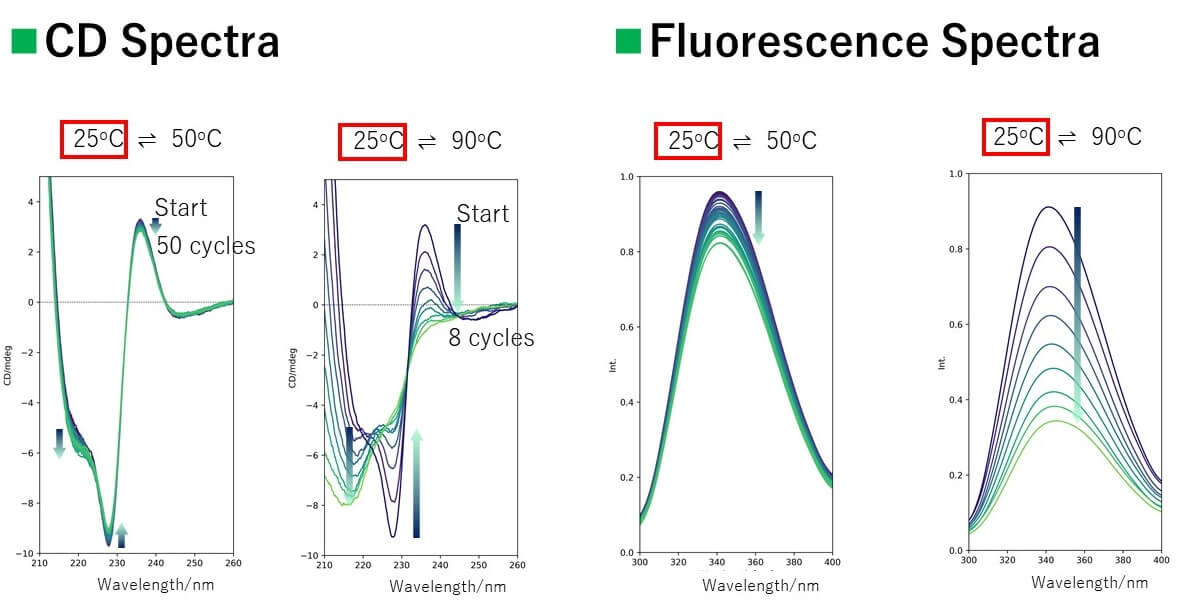
Results
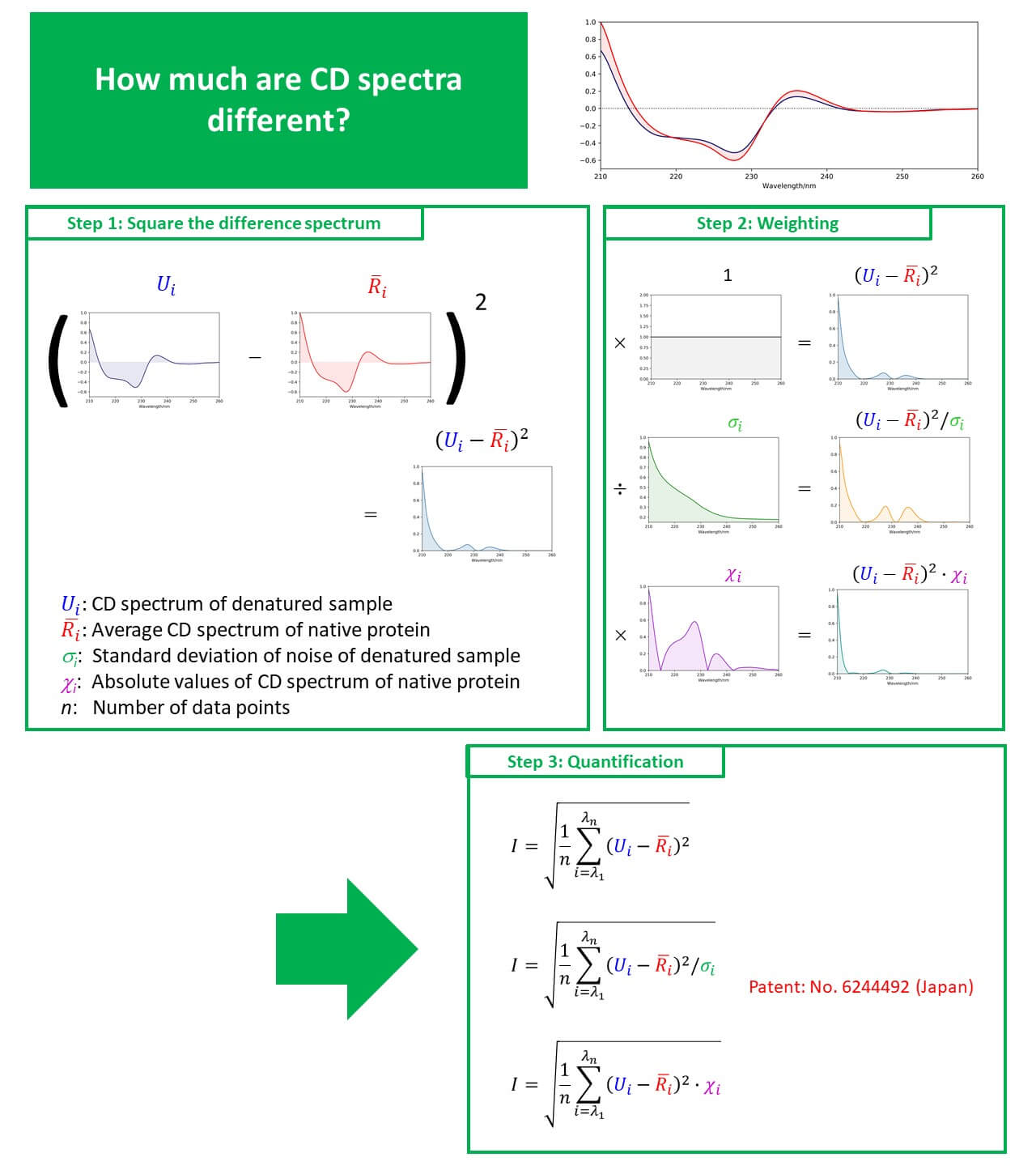
Calculation results
Conclusion
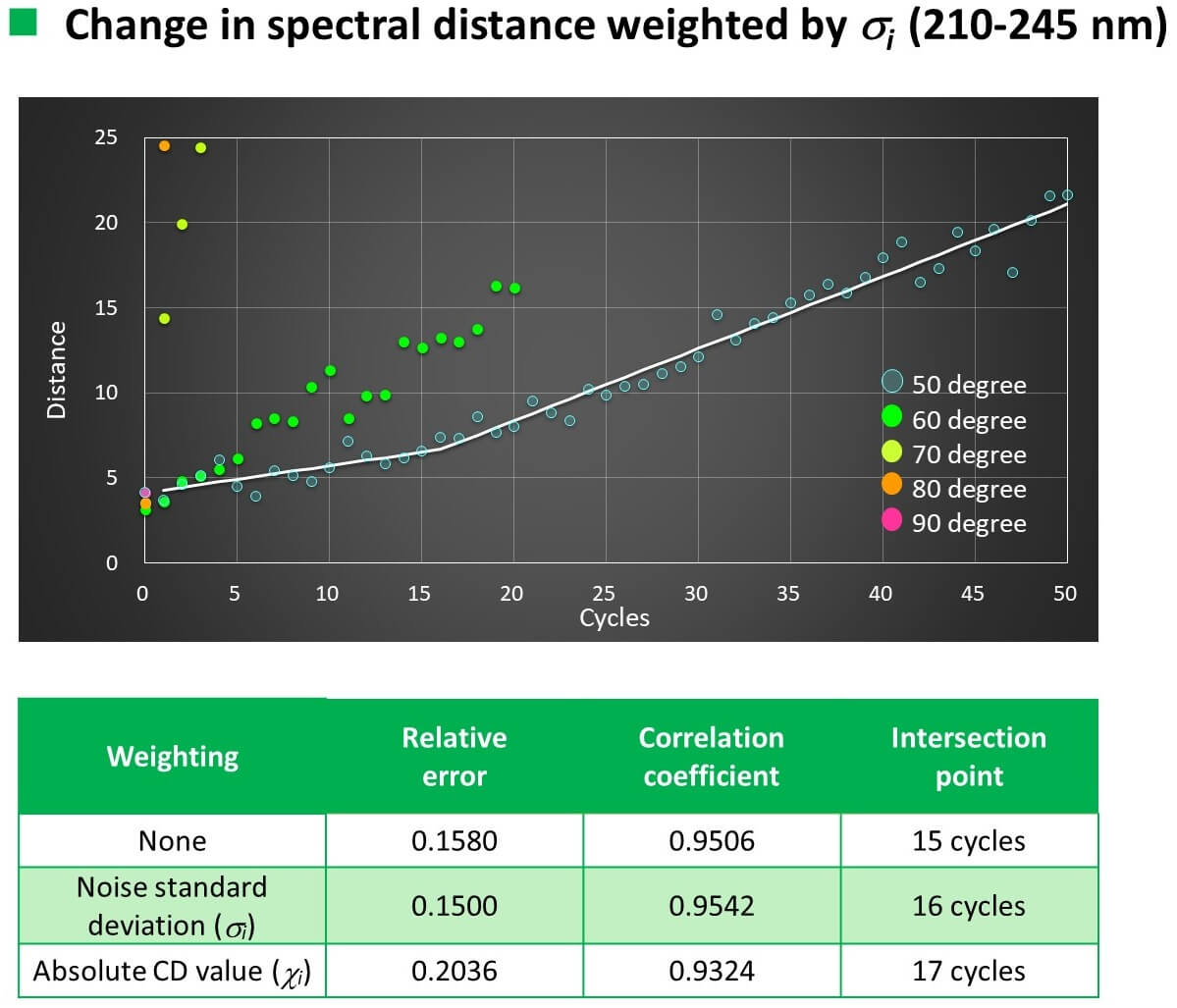
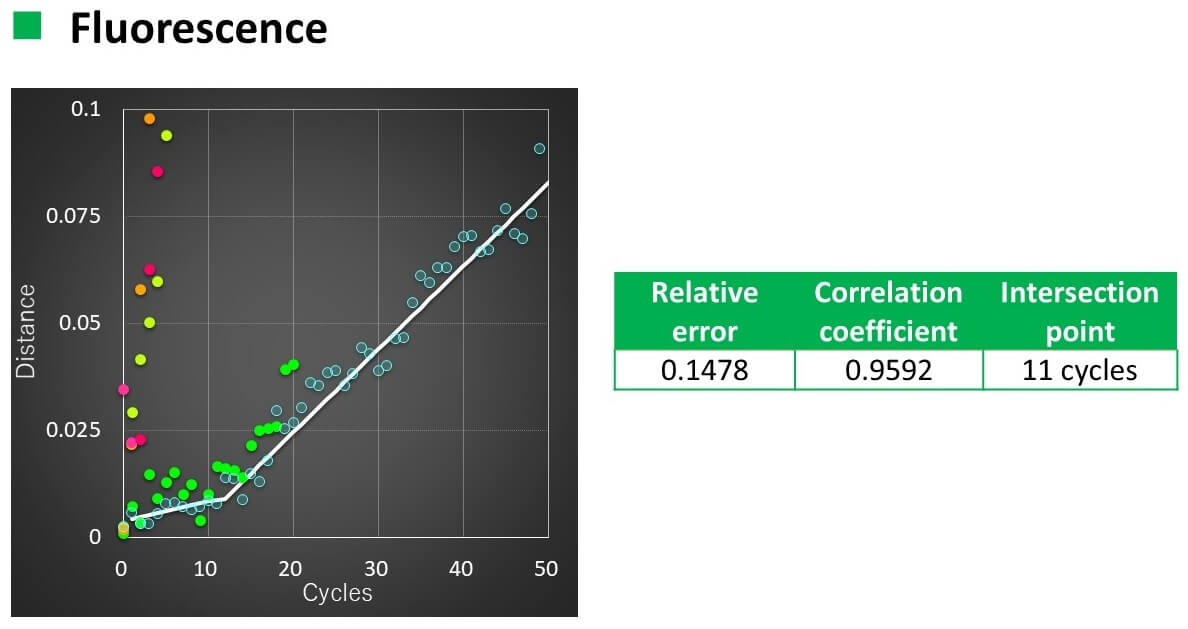
- The relationship between the number of temperature increase/decrease cycles and the spectral distance could be linearly approximated with a correlation coefficient of 0.93 to 0.96.
- As the result of examining the weighting function based on the Euclidean distance method, the highest accuracy was achieved using the noise standard deviation.
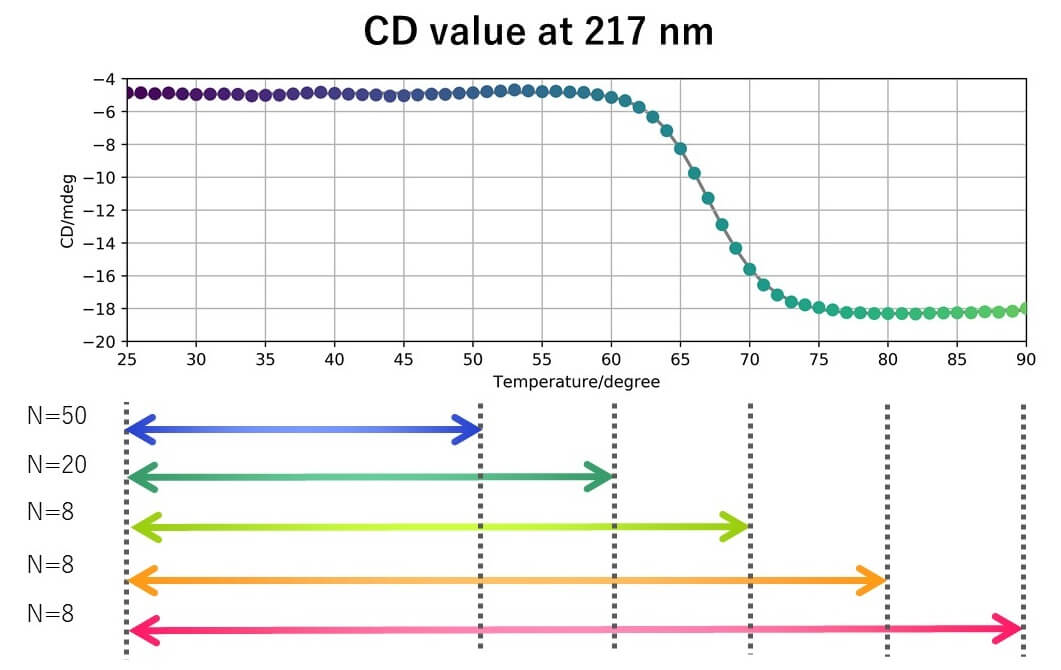
- When VHH was heated to 50 °C, the slope of the straight line changed after 16 cycles for CD and 11 cycles for FL.
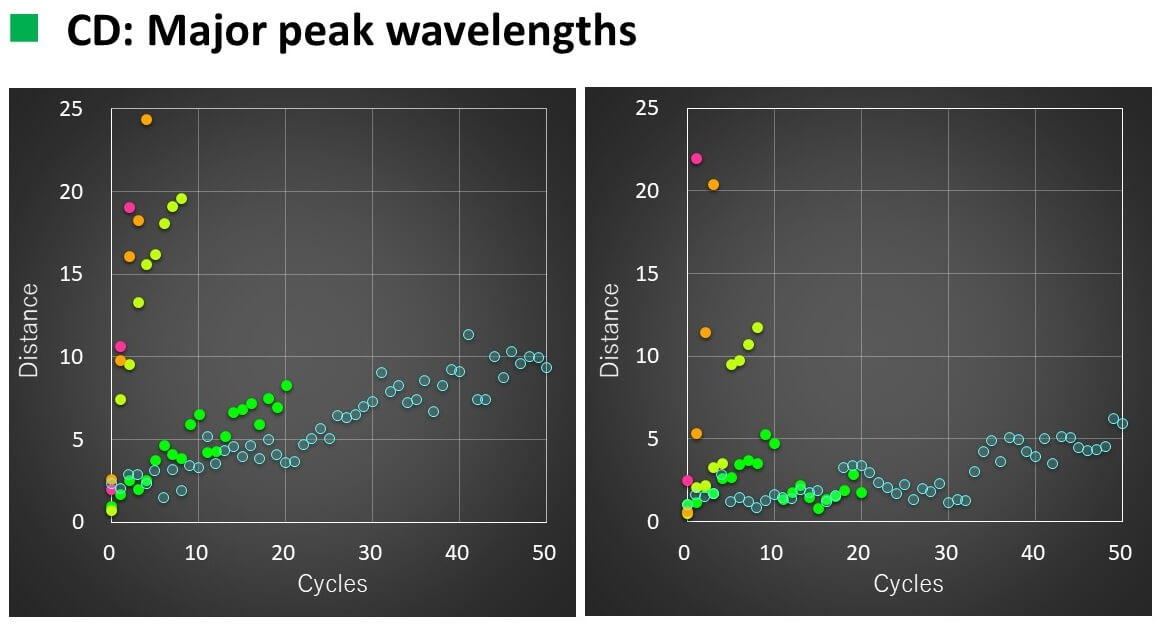
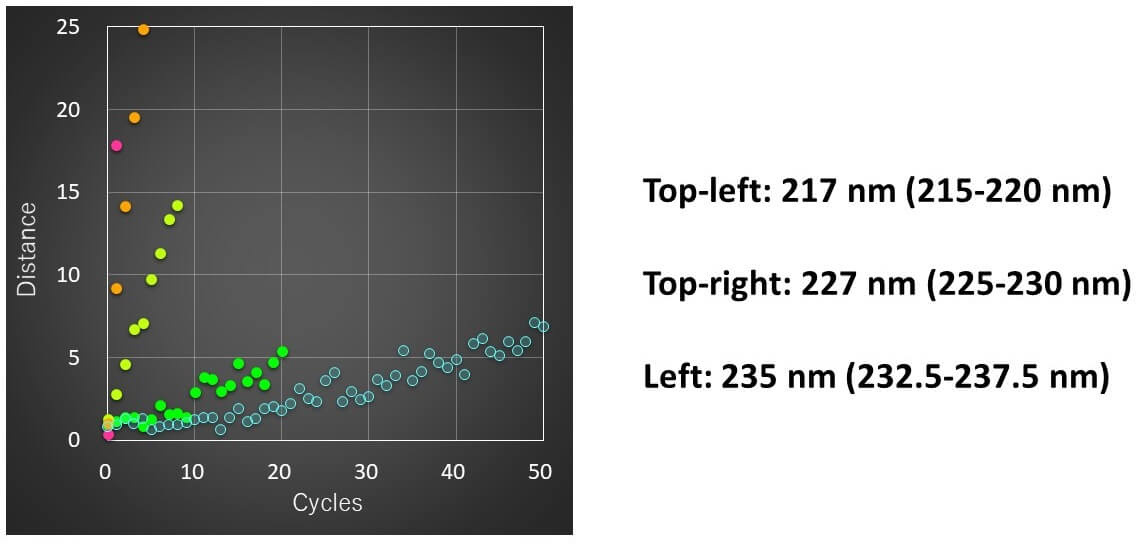
- The spectral distance showed the largest change for the CD peak around 217 nm, which is generally assigned to b-sheets.
References
Poster session at the 46th International Symposium on Nucleic Acids Chemistry.
Satoko Suzuki1, Emina Ikeuchi2, Taiji Oyama1, Yasuo Horiguchi1, Koushi Nagamori1, Hiroshi Nakayama3, and Kouhei Tusmoto2
1 JASCO Corporation, Tokyo, Japan,
2 School of Engineering and Institute of Medical Science, The University of Tokyo, Tokyo, Japan,
3 Tokyo University of Science, Tokyo, Japan