Introduction
Fluorescence and CD spectroscopy are complementary techniques in the structural analysis of proteins. Fluorescence spectroscopy provides information about the protein’s aromatic amino acid residues, while CD spectroscopy can elucidate conformational changes occurring along the protein backbone. Additionally, the temperature-dependent measurements can provide important information regarding the thermal stability of a protein. This data can be used to calculate the denaturation temperature (Tm), enthalpy changes (ΔH), and entropy changes (ΔS) for biological samples.
Experimental
The CD and fluorescence spectra of lysozyme were measured at varying temperatures to determine the thermodynamic stability of the protein. 
J-1500 CD Spectrometer
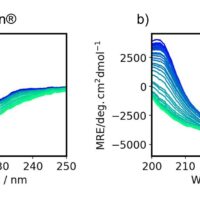
The CD spectra (Figure A) show a decrease in CD intensity as well as a shift in the negative peak at 208 nm to 203 nm with increasing temperatures. The fluorescence peak (Figure B) at 340 nm, reflecting tryptophan residues, is redshifted with increasing temperatures.
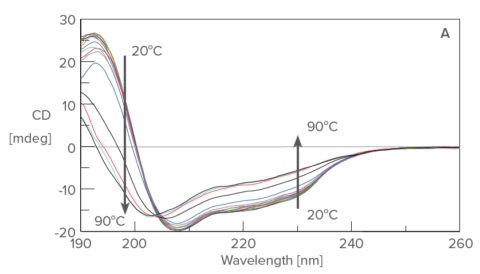
Temperature control CD measurement of lysozyme from 20 ºC to 90 ºC
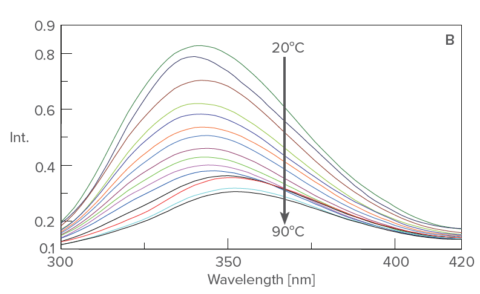
Temperature control fluorescence spectrum of lysozyme from 20ºC to 90ºC
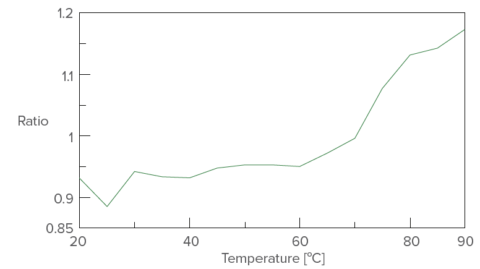
Temperature control plot of fluorescence intensity ratio (352 nm/340 nm)
These results indicate that with increasing temperatures, the native helical structure of lysozyme converts to random structures while the tryptophan residues move from the core of the protein to the surface.
Keywords
Bio medicinal product, Thermal denaturation, Secondary structure, Tertiary structure