Introduction
Stopped –flow CD measurement technique is well known method to analyze unfolding and refolding process of Protein and also observe complex forming reaction1, 2). Because Transition metal complex have typically absorbance in region from Visible to N-IR range.
We introduce this measurement about complex forming reaction of Nickel Sulfate and Rochelle Salt in using of high speed stopped flow systems consists of J-1700 and SFS-562.

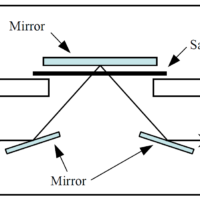
J-1700 CD spectrometer
Experimental
Measurement Condition
Syringe 1 : 0.24M Nickel Sulfate Solution
Syringe 2 : 0.36M Rochelle Salt Solution
Mixture ratio : 100 µl : 100 µl
Total flow rate : 5 ml/sec
Cell length : 2 mm
Measurement range : 720 nm (SBW5 nm, Data acquisition 75 times)
1000 nm (SBW10nm, Data acquisition 50 times)
Data pitch : 1 msec
Response : 2 msec
Results
CD spectra of mix solution including Nickel Sulfate and Rochelle Salt
Fig. 1 shows that CD spectrum of the mixed solution sample which consists of 0.24 M Nickel Sulfate solution and 0.36 M Rochelle Salt solution as 1:1 mixture ratio. It can show CD signal in broad range from UV/Vis to N-IR.
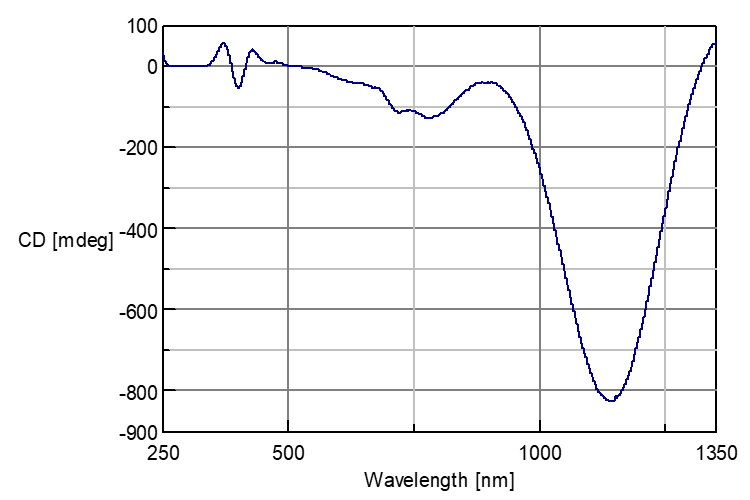
Fig. 1: CD spectra of mix solution including Nickel Sulfate and Rochelle Salt (Cell path :10 mm)
Stopped- Flow measurement
The complex forming process about the above sample is measured with CD stopped flow system. Following Fig.2 shows the CD spectra in NIR-region 720 nm and Fig.3 shows the one in N-IR region 1000 nm.
It shows in Fig.4 that complex forming reaction is finished within 100msec and shapes of both data in 720 nm, 1000 nm is match after data normalization, which mean both data in 720 nm and 1000 nm indicates same reaction process.
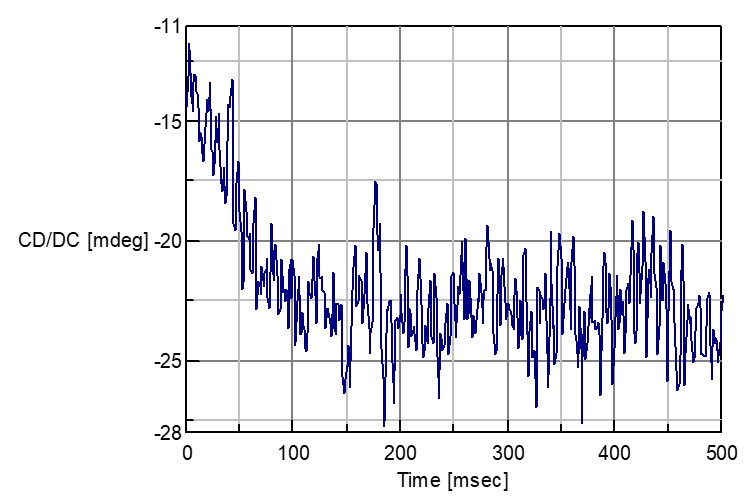
Fig. 2: Complex forming reaction of Nickel Sulfate and Rochelle salt in 720 nm.
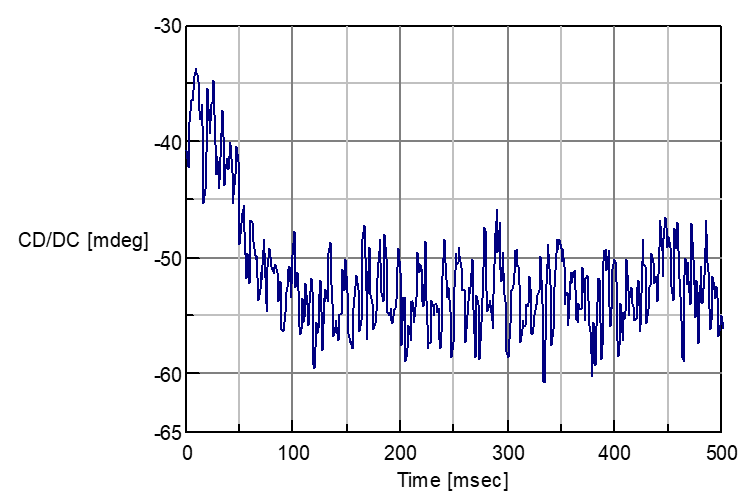
Fig. 3: Complex forming reaction of Nickel Sulfate and Rochelle salt in 1000 nm.
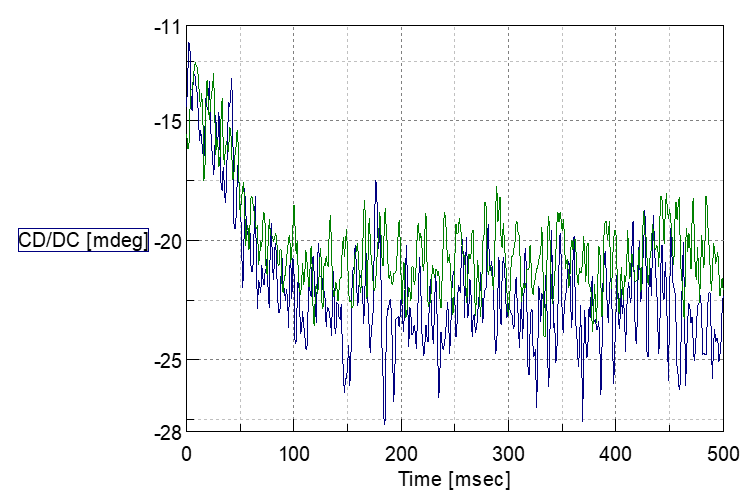
Fig. 4: Stopped flow data comparison between 720 nm (Blue) and 1000 nm (Green)
References
(1) Hiroyuki Miyake, Hideki Sugimoto, Hitoshi Tamiaki and Hiroshi Tsukube, (2005) Chem. Commun., 4291-4293
(2) Hiroyuki Miyake, Hiroshi Kamon, Ikuko Miyahara, Hideki Sugimoto, and Hiroshi Tsukube, (2008) J. Am. Chem. Soc., 130, 792-793