Introduction
Kinetic reaction analyses of chemical reaction are very important for studies of the reaction mechanisms and the factors which influence such reactions. Reaction analyses utilizing the vibrational spectroscopy are also efficient to investigate the structures of compounds and the reaction mechanisms. Chemical reaction analyses utilizing FT/IR in general can handle the reaction in a few or a couple of 10 minutes. This time polymerization process of quick-drying glue is measured by rapid scan (10 Hz).

FT/IR-6600 FTIR spectrometer
Experimental
Quick-drying glue was dripped on KBr plate, sandwiched with another plate and expanded, and the one of KBr plates was taken off. Transmittance method was applied for the measurement. After the measurement, five interferogram data were integrated each time, and Fourier transformed. In addition, the interferogram data in 0 – 10 seconds were Fourier transformed without integration.
Measurement conditions
Measurement time: 1 min.
Detector: MCT
Resolution: 16 cm-1 (fixed)
Data acquisition: 10 Hz (fixed)
Results
Figure 1 shows time-course spectra (five interferogram integrated each time) as curing process of superglue in a minute. Absorbance of 1257 cm-1 peak was increased in accordance with curing.
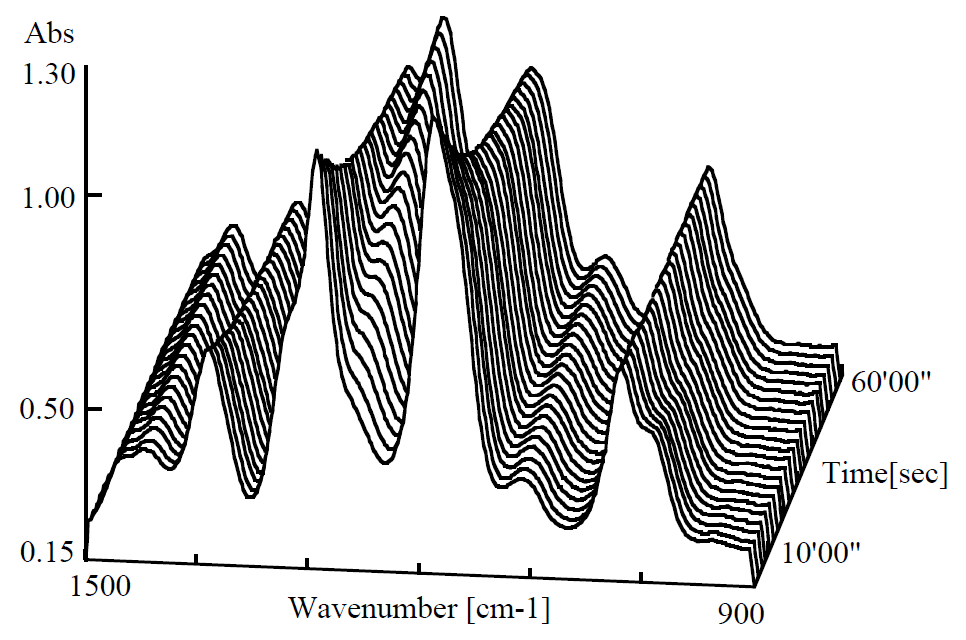
Figure 1. Curing process of quick-drying glue (0 – 60 sec.)
Figure 2 shows time scan spectra (non-integration) in 10 seconds. Absorbance of peak in the vicinity of 1257 cm-1 was little increased.
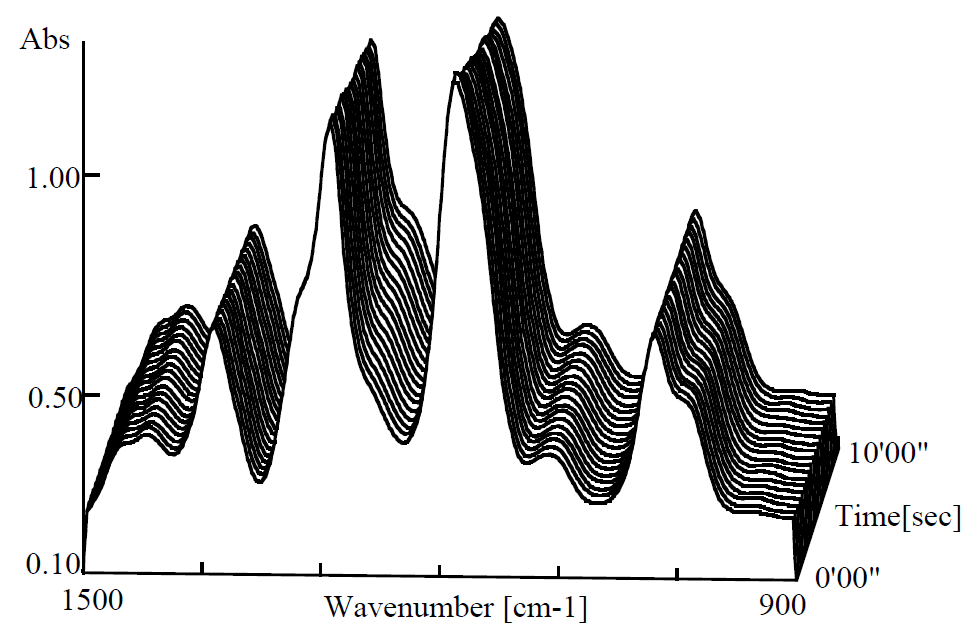
Figure 2. Curing process of quick-drying glue (0 – 10 sec.)
Figure 3 shows IR spectra of 0 and 60 seconds respectively. It is generally known that curing mechanism of syanoacrylate occurs due to the additive polymerization of double bond. Based on this fact, it is considered that absorption peak of 2998 cm-1 is attributed to CH, 1739 cm-1, to C=O, 1442 cm-1, to –CH2-CO- (strong) and CH2-C=C (weak), 1292 cm-1, to C=C-COO and 1257 cm-1, to CH3-COO-R.
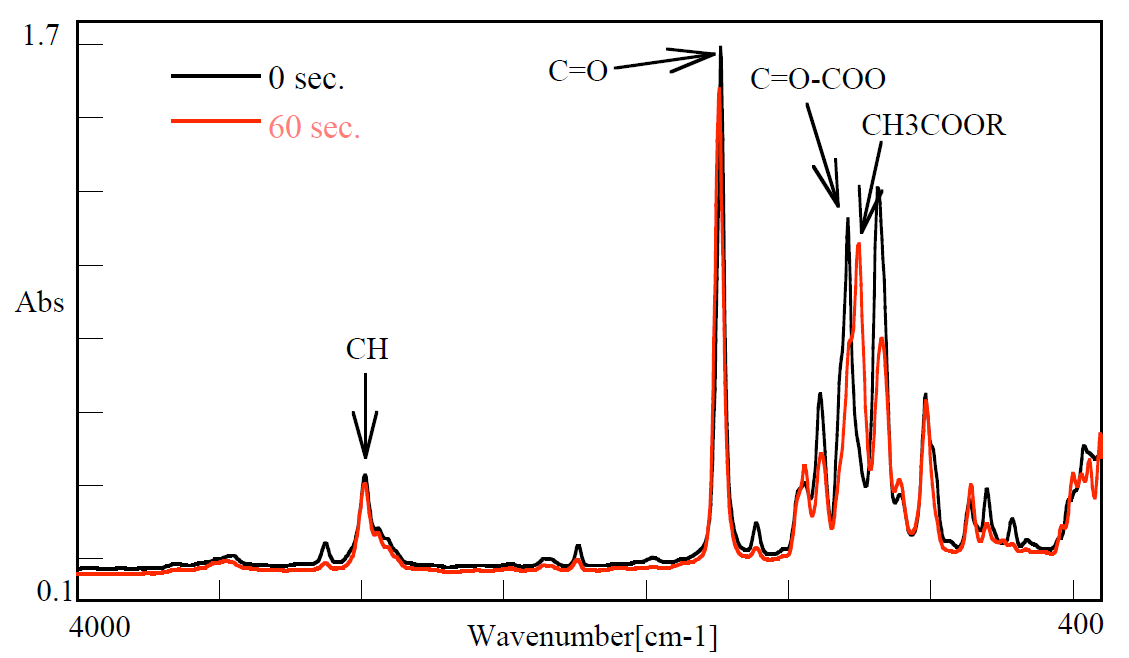
Figure 3. Spectra of quick-drying glue at 0 and 60 seconds
Since the shape of absorption peak of 2998 cm-1 attributed to CH was not changed with time, the time-course curves of the absorption peaks of 1292 and 1257 cm-1 were calculated based on 2998 cm-1 as thickness correction reference (Figure 4). This results indicates that half of the reaction finished in around 20 seconds and the polymerization of other half was processed slowly in more than 40 seconds.
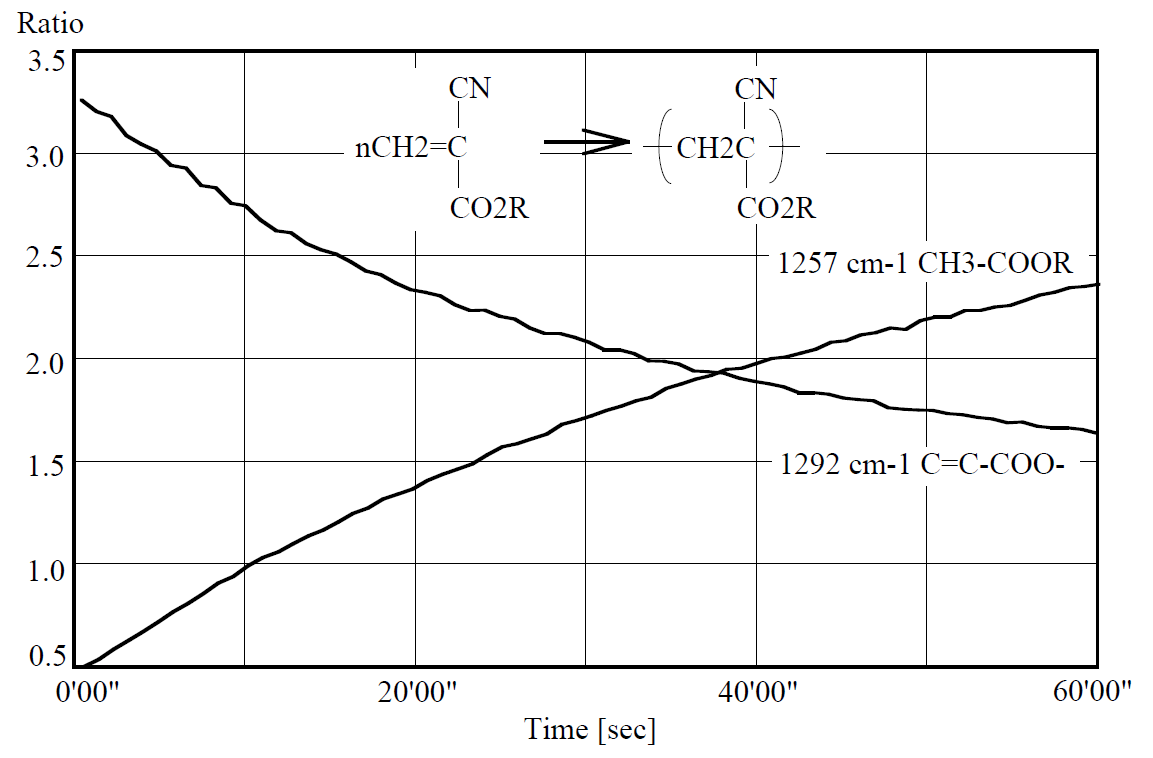
Figure 4. Time-course curves of 1257 and 1292 cm-1