Introduction
Sterols can be derived from plant material – plant sterol (phytosterol), or from animals – animal sterol (zoosterol). A commonly occurring zoosterol is cholesterol, this molecule is an important component found in animal cell membranes.
Recent research has revealed that there is no significant relationship between the consumption of dietary cholesterol and serum (blood) cholesterol levels. However, in general, dietary patterns that are lower in cholesterol are recommended for reducing the risks of cardiovascular disease.
Phytosterol inhibits the absorption of cholesterol by internal organs, so it helps in reducing physiological cholesterol levels. Therefore, the analysis and quantitation of sterols is important in food production and quality control.
This article illustrates the analysis of several different sterol standard samples (four types of phytosterols and cholesterol (shown in Figure 1)) using a UHPLC column with 1.9 μm particles. Additionally, a mayonnaise containing phytosterol and a conventional mayonnaise have also been analyzed.
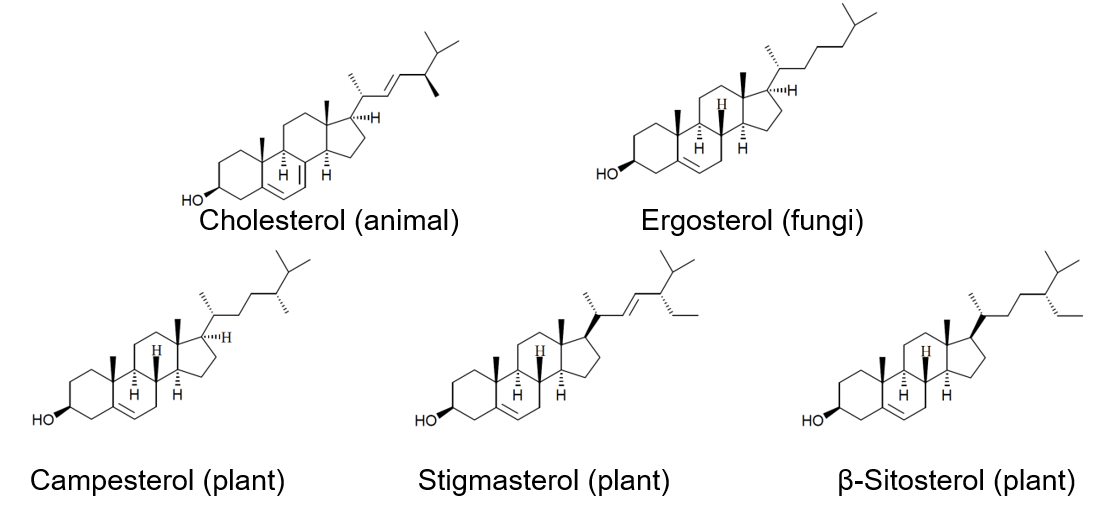
Figure 1. Structural formula of sterols
Experimental
Experimental Condition
Column: Unifinepak C18 (2.0 mmID x 150 mmL, 1.9 µm)
Eluent : A; Acetonitrile/Water (95/5), B; THF
Flow rate: 0.5 mL/min
Column temp.: 40 ºC
Detection: UV detection (Wavelength: 278 nm)
Injection volume: 1 µL
Standard Sample: Ergosterol, Cholesterol, Campesterol, Stigmasterol, β-Sitosterol (0.5 mg/mL each)
Keywords
Sterol, UHPLC, Phytosterol, Cholesterol, Unifinepak C18 column, UV detector, HPLC
Results
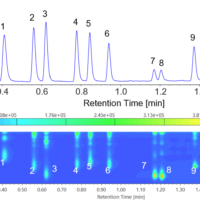
Figure 2 shows the chromatogram of sterol standard sample. THF solutions of 10 mg/mL sterols were prepared as stock solutions, and diluted with acetonitrile to prepare 0.5 mg/mL standard samples.
As shown in Figure 2, a high resolution UHPLC column (2.0 mm ID x 150 mm L, 1.9 μm particles) completely separates campesterol (peak 3) and stigmasterol (peak 4) with baseline resolution.
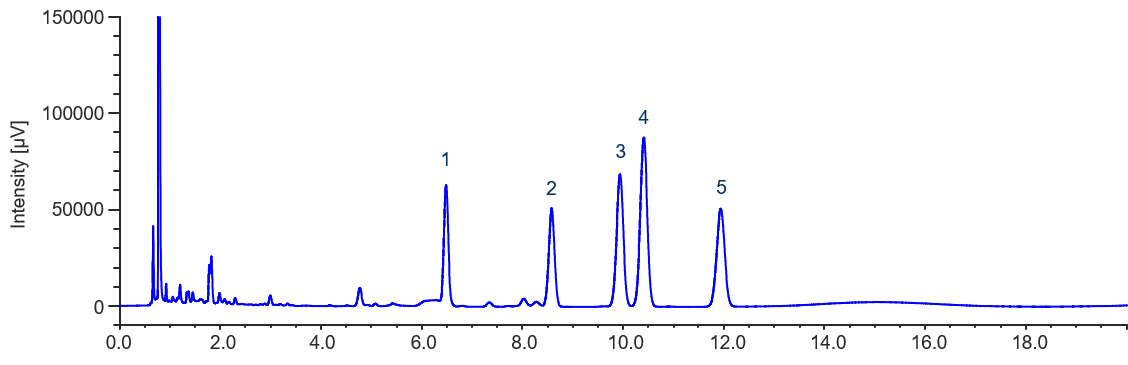
Figure 2. Chromatogram of sterol standard sample (1. Ergosterol, 2. Cholesterol, 3. Campesterol, 4. Stigmasterol, 5. β-Sitosterol)
Next, two mayonnaise samples were measured; the first contained phytosterol, and the second a more commonly used mayonnaise. Figures 3 and 4 show the pretreatment procedure of each sample. As shown in the Figures 3 and 4, the pretreatment procedures are different for each sample.

Figure 3. Pretreatment procedure of a sample containing phytosterol

Figure 4. Pretreatment procedure of common mayonnaise
As shown in Figure 5, UHPLC could be used to clearly identify several different sterols – phytosterol; campesterol, stigmasterol, and β-sitosterol.
In this measurement, components which are strongly retained are included in this sample, these are difficult to remove during pre-treatment. Therefore, the column must be flushed with THF (B solvent) between sample measurements.
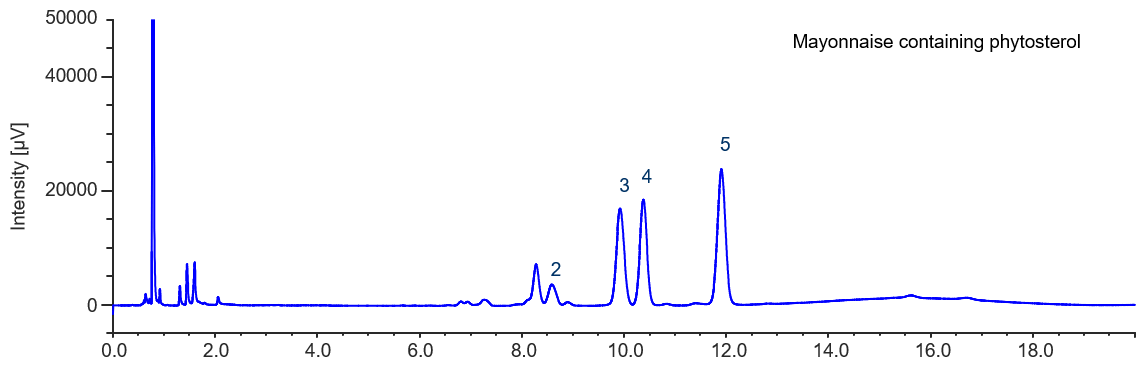
Figure 5. Chromatogram of sample containing phytosterol (2. Cholesterol, 3. Campesterol, 4. Stigmasterol, 5. β-Sitosterol)
During pretreatment of the sample of common mayonnasie, dilution with acetonitrile was not performed. Therefore, it should be noted that the standard sample concentration in Figure 6 is ten times higher than that in Figure 5.
Figure 6 indicates that a low level of phytosterol is present in the common mayonnaise. This may be due to plant oil being used in the production of this mayonnaise.
These results show that the UHPLC is a fast, accurate and reliable method for the analysis of sterols.
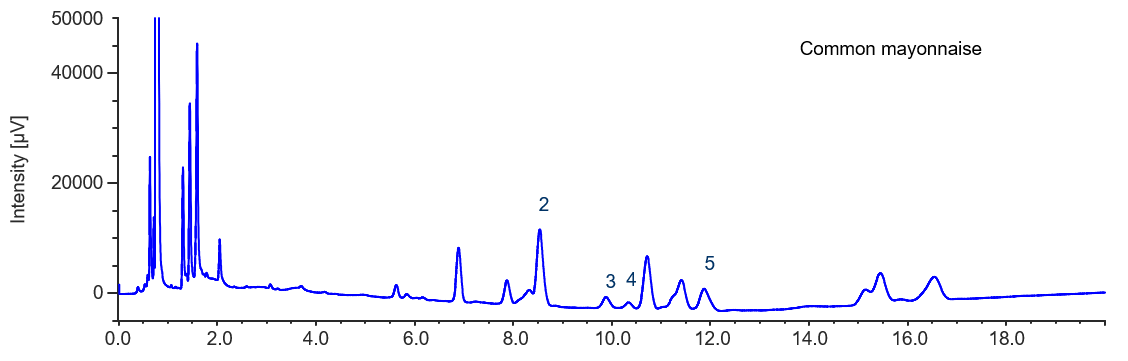
Figure 6. Chromatogram of common semi-solid dressing (2. Cholesterol, 3. Campesterol, 4. Stigmasterol, 5. β-Sitosterol)