Introduction
Polycyclic aromatic hydrocarbons (PAHs) are chemicals generated when fuels are incompletely combusted, which are contained in industrial and car exhaust and are known as the main source of air pollution. Some of them are categorized as carcinogens and these compounds are subjected to the regulations of governmental agencies such as EPA (Environmental Protection Agency) in U.S.A.
In this application, PAHs in diesel exhaust particulate matter are measured using Ultra High-performance Liquid Chromatography (UHPLC) with PDA detection.

LC-4000 UHPLC system
Experimental
Chromatographic conditions
Column: ZORBAX Eclipse PAH (2.0 mmID x 50 mmL, 1.8 µm)
Eluent A: Water
Eluent B: Acetonitrile
Gradient condition: (A/B), 0 min (60/40) -> 5.0 min (0/100) -> 6.5 min (0/100) -> 6.55 min (60/40) 1 cycle; 9.0 min
Flow rate: 0.6 mL/min
Column temp.: 30ºC
Wavelength: 200-500 nm
Injection volume: 1 µL
Standard sample: PAH mixture (EPA 610 16 Mix Solution 20 ppm each in acetonitrile)
Keywords
UHPLC, diesel exhaust, polycyclic aromatic hydrocarbon, PAHs, 1.8 µm packing material, C18 column, PDA detector
Results
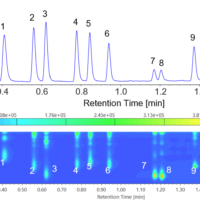
Figure 1 shows the chromatogram of the PAH standard mixture (EPA 610) and the contour plot. 16 compounds were clearly separated within 6 minutes.
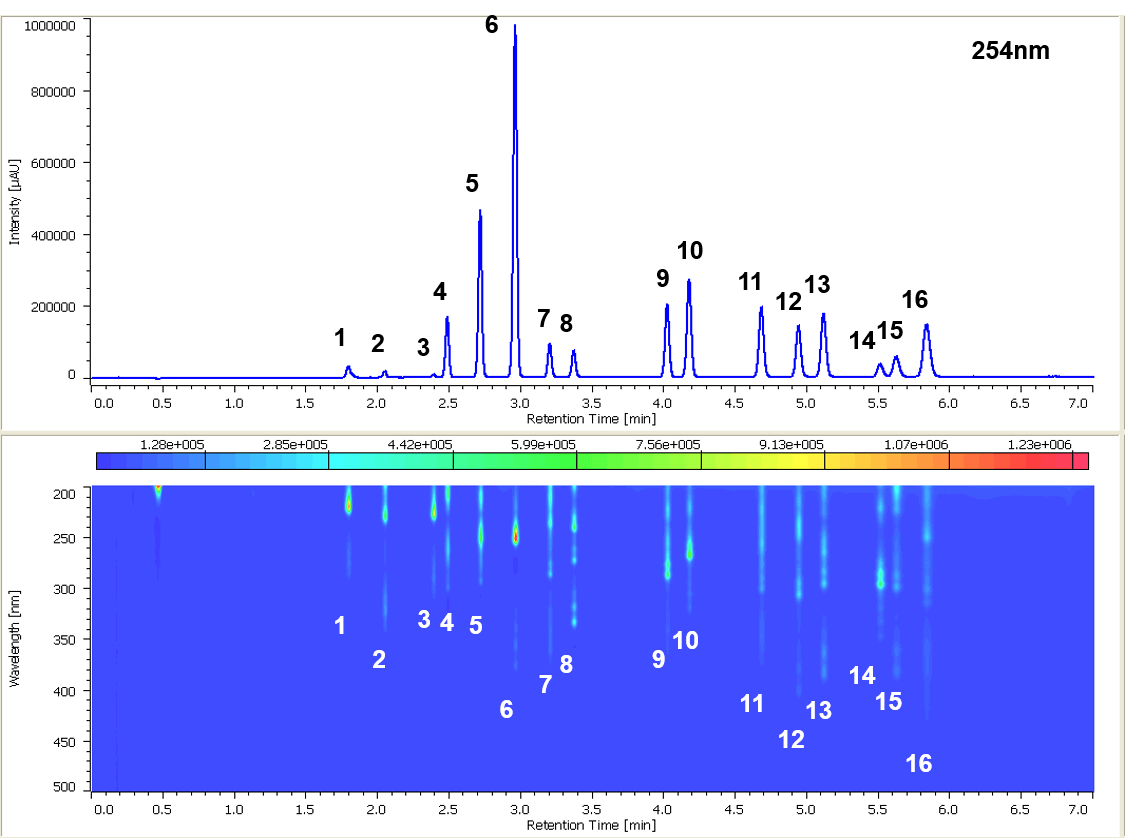
Figure 1. Chromatogram of the PAH standard mixture (1: Naphthalene, 2: Acenaphthylene, 3: Acenaphthene, 4: Fluorene, 5: Phenanthrene, 6: Anthracene, 7: Fluoranthene, 8: Pyrene, 9: Benzo[a]anthracene, 10: Chrysene, 11: Benzo[b]fluoranthene, 12: Benzo[k]fluoranthene, 13: Benzo[a]pyrene, 14: Dibenzo[ah]anthracene, 15: Benzo[ghi]perylene, 16: Indeno[123-cd]pyrene)
Figure 2 shows the chromatogram and the contour plot of the sample solution that is extracted from diesel exhaust particulate matter with Soxhlet extraction. It was confirmed from the spectral information that PAHs were detected.
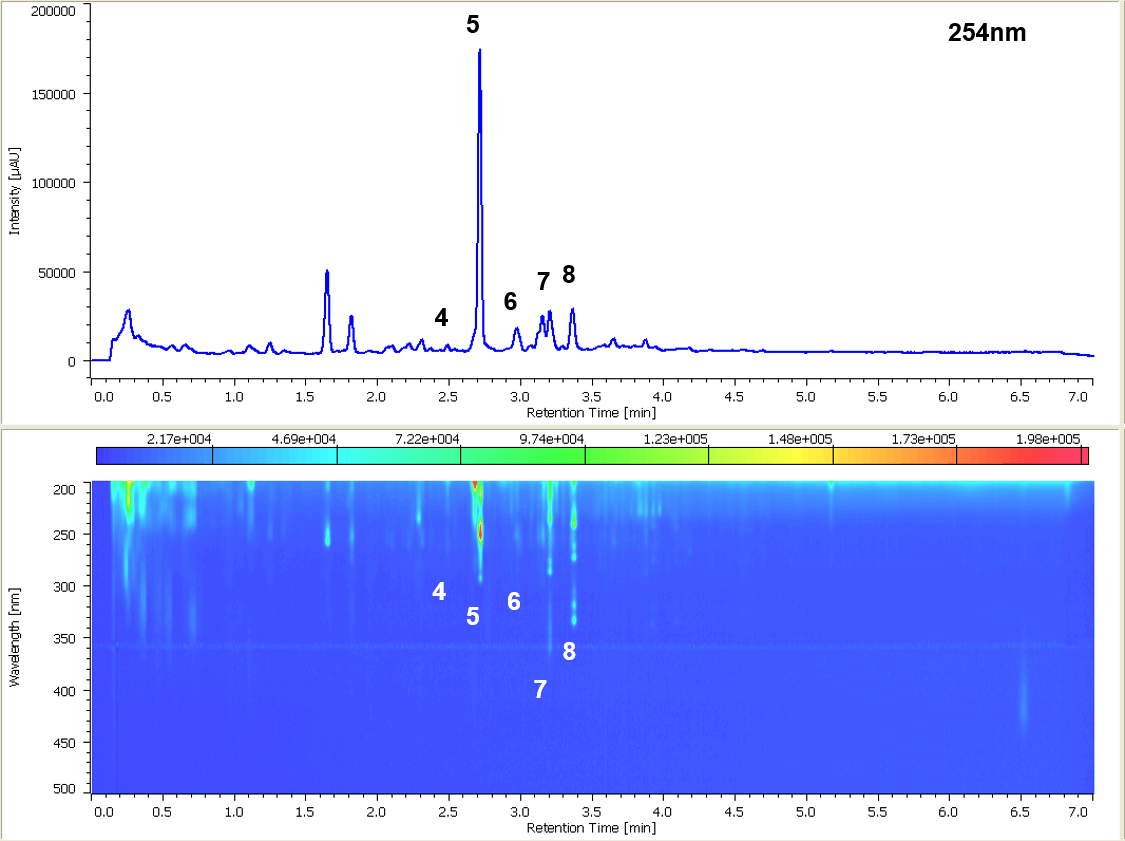
Figure 2. Chromatogram of extract from diesel exhaust particulate matter. The peak numbers and corresponding compounds are the same as in figure 1.
Sample preparation
Sample solution was filtrated using 0.2 um membrane filter after Soxhlet extraction.