Introduction
Anionic surfactants are active ingredients in synthetic detergents and their analysis is one of the water-quality test items specified in Japan’s Water Supply Act, as they are indicators of pollution caused by contamination from factory effluents and domestic wastewater. HPLC combined with fluorescence detection is used to measure levels of anionic surfactants in water (Notification No. 261 of the Ministry of Health, Labour and Welfare, 2003, Appendix 24).1 In this method, sodium alkylbenzenesulphonate (ABS), with carbon atoms in the alkyl chain at numbers between 10 and 14, is the target of the measurement, with a standard value of 0.2 mg/L for the sum of the five components. When carrying out water quality testing, it is necessary to measure down to a concentration of 1/10th of the standard value and to ensure that the measurement precision error at this concentration is less than 20%.
A typical C18 column (modified group: octadecyl group) can recognize the branched state of carbon chains so that ABS molecules with an identical number of carbon atoms are separated based on their branching state and produce multiple peaks. In this application note, we report the separation of anion surfactants using a C8 column (modified group: octyl group), which does not distinguish between branching states but instead produces a different elution peak depending on the number of carbon atoms in the chain, together with highly sensitive analysis using a fluorescence detector.
Note that a peak due to toluene may also be detected in the analysis of anionic surfactants. This can occur if measurement equipment, laboratory instruments, and the laboratory room are also used for analysis of nonionic surfactants (Appendix Table 28-2), which is another water-quality test item (toluene is used for sample pretreatment in this analysis). In this application note, we describe how peaks associated with anionic surfactants and toluene can be separated and detected.
LC-4000 series HPLC system
Experimental
<Instruments>
Pump: PU-4180*
Autosampler: AS-4050*
Column oven: CO-4060
FP detector: FP-4025*
* with option units
<Conditions>
Column: Inertsil C8-4 (4.6 mmI.D. x 150 mmL, 5 µm)*
Eluent: Acetonitrile/0.1 mol/L sodium perchlorate in water (58/42)
Flow rate: 1.0 mL/min
Column temp.: 40 ºC
Wavelength: Ex. 221 nm, Em. 284 nm, Gain x 10
Inj. volume: 5 µL
Standard: Mixture of anionic surfactants in methanol
(1.0 mg/L, 2.0 mg/L, 5.0 mg/L, 10 mg/L, 25 mg/mL each)
* Inertsil is a trademark or registered mark of GL Sciences Inc.
<Structure>

Keywords
anionic surfactant, sodium alkylbenzenesulfonate, water-quality standard, C8 column, fluorescence detector
Results
Since the sample used for water testing is first concentrated 250 times by solid-phase extraction, the range of the calibration curve reflects this higher concentration. Table 1 shows the concentration of each component calculated from the standard value and that following condensation by solid-phase extraction. In this experiment, the minimum and maximum concentrations of each component were set at 1 mg/L and 25 mg/L, respectively, and calibration curves were created by performing three repeat measurements at five different concentrations. The results show good linearity with a correlation coefficient of r = 0.9996 or better for all components.
Table 1 Concentration range for calibration curve calculated from standard values for five anionic surfactant components
| Concentration at sampling [mg/L] | Concentration after 250 times condensation [mg/L] | ||
| Standard value | Sum of 5 components | 0.2 | 50 |
| 1 component | 0.04 | 10 | |
| 1/10 of standard value | Sum of 5 components | 0.02 | 5 |
| 1 component | 0.004 | 1 | |
| Calibration range | Sum of 5 components | 0.02-0.5 | 5-125 |
| 1 component | 0.004-0.1 | 1-25 | |
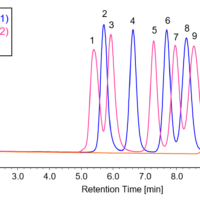
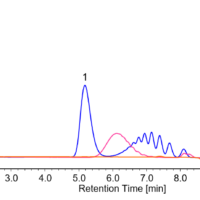
Figure 1 shows chromatograms for a standard mixture of anionic surfactants at each concentration when the calibration solution was measured (the second time). Figure 2 shows a chromatogram of a blank (methanol) measured immediately after measuring a standard sample with the maximum concentration. The results confirm that no carryover above the minimum concentration in the calibration curve occurs.
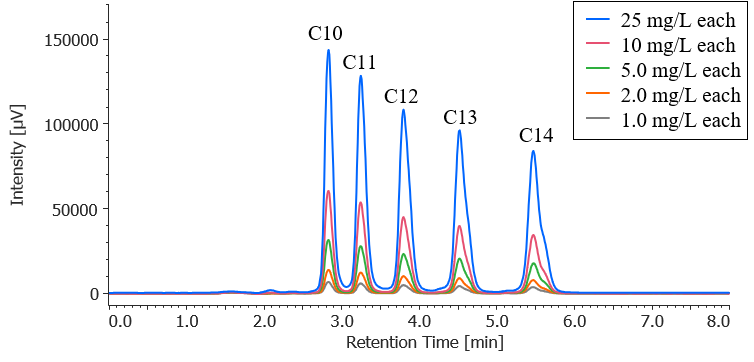
Fig. 1 Chromatograms for standard mixture of anionic surfactants (second measurement of calibration solutions)
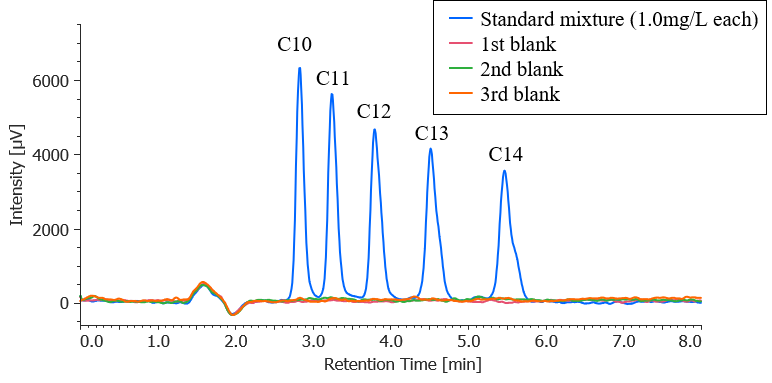
Fig. 2 Blank chromatograms for carryover check (chromatogram for standard mixture with 1 mg/L each is overlaid)
Table 2 shows the quantification results for the standard mixture of anionic surfactants at each concentration based on the calibration curve, and the calculated accuracy of the observed values relative to the theoretical concentrations. For all components and concentrations, the accuracy was found to be 80-120% of the theoretical concentration.
Table 2 Accuracy of measured concentrations relative to theoretical values for standard mixtures of anionic surfactants
| Theoretical concentration [mg/L] | Accuracy [%]* | ||||
| C10 | C11 | C12 | C13 | C14 | |
| 1.0 | 90 | 91 | 91 | 91 | 93 |
| 2.0 | 106 | 106 | 106 | 107 | 105 |
| 5.0 | 103 | 103 | 103 | 103 | 102 |
| 10 | 101 | 101 | 101 | 100 | 100 |
| 25 | 99 | 99 | 99 | 99 | 99 |
* The average of the quantitative values obtained by repeat measurements (n = 3) is used for the calculation.
Figure 3 shows the results of five repeat measurements of a standard sample with a concentration of 1/10 of the standard value. The reproducibility of the peak area for each component is shown in Table 3. The results confirm that the measurement precision error is less than 20% for all components.
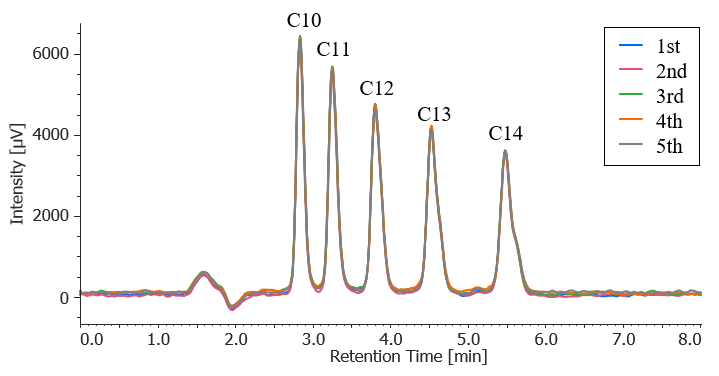
Fig. 3 Repeat measurement results for standard mixtures of anionic surfactants (1.0 mg/L each, n = 5)
Table 3 Reproducibility for standard mixture of anionic surfactants (1.0 mg/L each, n = 5)
| Number of injection | Peak area [µV・sec] | ||||
| C10 | C11 | C12 | C13 | C14 | |
| 1 | 45725 | 45957 | 44156 | 43397 | 41862 |
| 2 | 46866 | 45890 | 42931 | 41377 | 42613 |
| 3 | 46295 | 45836 | 43390 | 43309 | 40751 |
| 4 | 46030 | 45724 | 43264 | 42321 | 42026 |
| 5 | 47357 | 46875 | 45700 | 44869 | 41251 |
| Average | 46454 | 46056 | 43888 | 43055 | 41701 |
| SD | 656 | 465 | 1108 | 1306 | 719 |
| RSD[%] | 1.41 | 1.01 | 2.52 | 3.03 | 1.72 |
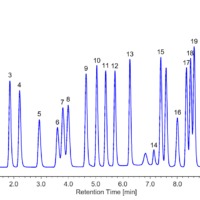
Figure 4 shows a chromatogram for a standard mixture of anionic surfactants (1.0 mg/L each) with toluene added at 1 mg/L. The toluene-derived peak appears between the C13 and C14 peaks, and good separation is achieved. Therefore, reliable analysis of anionic surfactants can be achieved even in a laboratory environment in which nonionic surfactant analyses using toluene are performed.
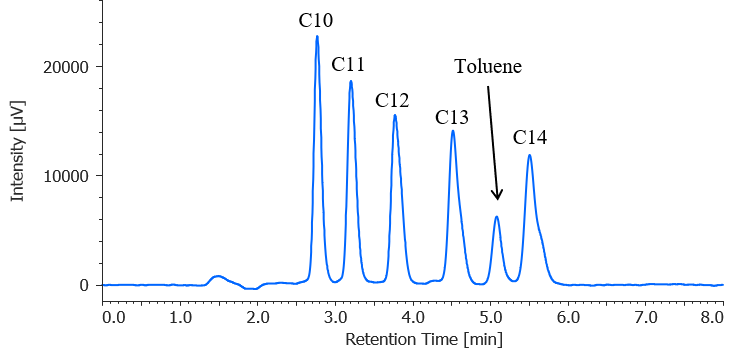
Fig. 4 Chromatogram for standard mixture of anionic surfactants (1 mg/L each) with toluene added at 1 mg/L
References
1.Ministry of the Environment: “Suishitsukijun ni kansuru shourei no kitei ni motoduki kouseiroudoudaijin ga sadameru houhou (in Japanese).” <https://www.env.go.jp/content/900547779.pdf>, (accessed 2025. 3. 10).