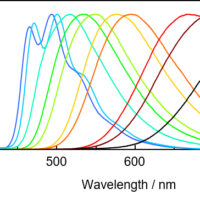Fluorescence quenching
Quenching by dissolved oxygen
The fluorescence intensity of naphthalene and anthracene decreases with time due to dissolved oxygen. To prevent such quenching, remove dissolved oxygen by N2 gas replacement or vacuum deaeration.
Thermal quenching
In general, the fluorescence intensity decreases with increasing temperature. Therefore, when performing fluorescence measurements, temperature management is more critical than in the case of absorption measurements.
Concentration quenching
For many fluorescent solutions, the fluorescence intensity decreases at high concentrations. This is similar to internal shielding , but the mechanism is different and is due to intermolecular interactions. Conversely, some solutions, such as pseudocyanine aqueous solutions, emit fluorescence only at high concentrations.
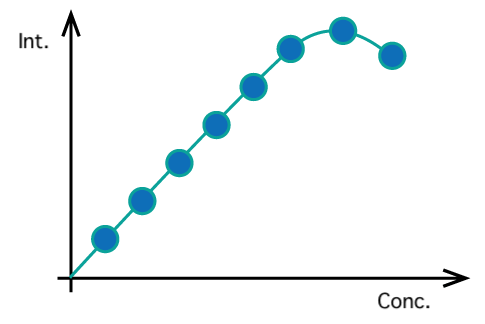
Fig. 9 Concentration quenching
Quenching by impurities
When the purity of the reagent or solvent used for fluorescence measurements is low, the measurement results may be inaccurate due to quenching by impurities due to reaction with the reagent.
Internal shielding effect
In the case of a high-concentration sample, the excitation light is strongly absorbed near the cell surface and cannot reach the interior of the cell. This is called an internal shielding effect, and the spectrum is distorted. Fig. 10 shows excitation spectra of a dilute solution and a concentrated solution of quinine sulfate. The excitation spectrum of the concentrated solution is a convolution of that of the dilute solution and the transmission spectrum of the concentrated solution.
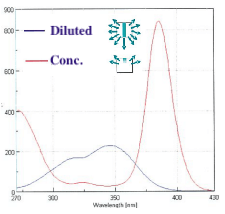
Fig. 10 Excitation spectrum of quinine sulfate solutions with different concentrations (Em: 450 nm)
Spectral correction
In UV/VIS spectroscopy, the photometric scale is standardized as 0-100 % transmittance. In fluorescence spectroscopy, there is no reference scale and the relative increase from 'dark' is measured. Additionally, the detected fluorescence intensity is the product of the sample's fluorescence intensity and an instrument function, which is similar to the baseline in UV/VIS spectroscopy. In order to obtain the true spectrum, it is necessary to correct the observed spectrum by removing the effect of the instrument function.