Introduction
In industrial fields such as food and chemistry, quantitative analysis is widely used in the development, acceptance testing, and quality control of products. UV-visible (UV-Vis) spectroscopy and infrared (IR) spectroscopy have been the leading methods for quantitative analysis. However, for quantitative analysis of aqueous solutions containing sugars such as glucose, generally speaking, UV-vis and IR spectroscopic methods are not suitable because sugar exhibits no absorption bands in the UV-vis region and water exhibits strong absorption in the IR region.1) This report describes an example of quantitative analysis of glucose in aqueous solution using Raman spectroscopy, which can analyze sugars and is not affected by water.
The Palmtop Raman spectrometer and the optional Cuvette holder were used for the measurements.
Cuvette holder
Since there is a proportional relationship between the Raman peak intensity and the concentration of a target component, quantitative analysis can be conducted based on the peak intensity. For quantitative measurements, it is important to set the sample in the same position each time because the laser focus position affects the intensity of the Raman signal. The cuvette holder and the supplied spacer allow for highly reproducible sample positioning and highly accurate focusing, making them useful for quantitative analysis.
In this experiment, a calibration curve for 0.5-10 wt% aqueous glucose solutions was prepared.
Experimental
Samples
Aqueous glucose solutions with concentrations of 0.5, 1, 2, 5, and 10 wt% (total of 5 samples)
Ultrapure water (reference)
System
Instrument: PR-1w Palmtop Raman Spectrometer
Accessories: PR-1-C Cuvette holder, Rectangular quartz cell with lid, light path length 10 mm
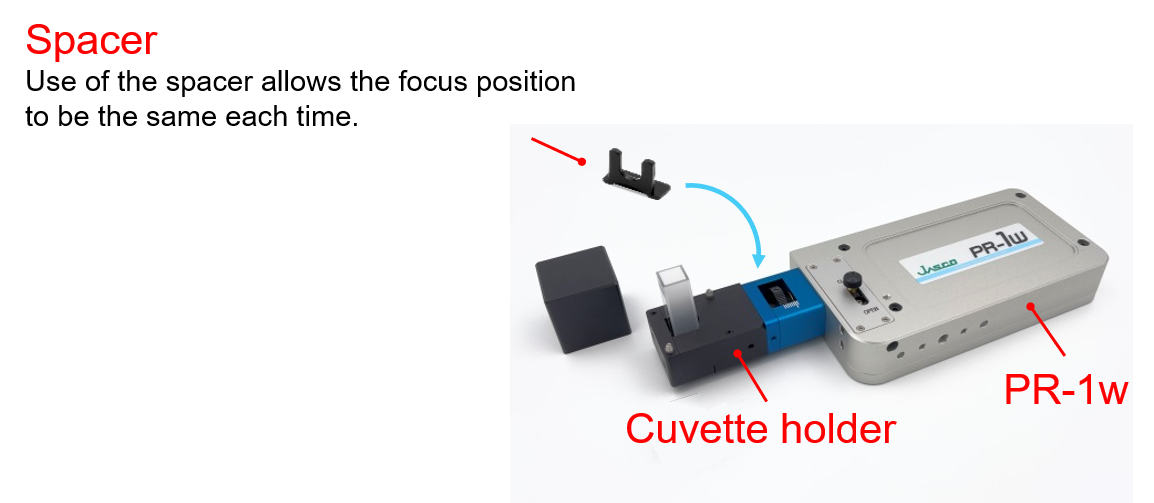
Fig. 1 Cuvette holder attached
Parameters
Excitation wavelength: 785 nm
Laser power output: 50 mW
Keywords
Quantitative analysis, aqueous solution, glucose, Palmtop Raman spectrometer, Raman spectroscopy
Results
Figure 2a shows spectra for five different sample concentrations and for ultrapure water. It can be observed that the intensity of the peak due to angular vibrations of water near 1630 cm-1 (pink shaded area) changes very little. However, the intensity in the blue shaded areas assigned to angular vibrations of -COC-glycosidic and -CH bonds (200 to 1500 cm-1), and stretching vibrations of –CH bonds (2800 to 3000 cm-1), which are associated with glucose, increases with increasing glucose concentration. Figure 2b shows a calibration curve created using the intensity of the peak near 1130 cm-1 for glucose concentrations of 0.5 to 10 wt%. The curve is seen to be highly linear with a correlation coefficient of R = 0.9999, indicating the effectiveness of this system for evaluating aqueous glucose solutions in this concentration range.
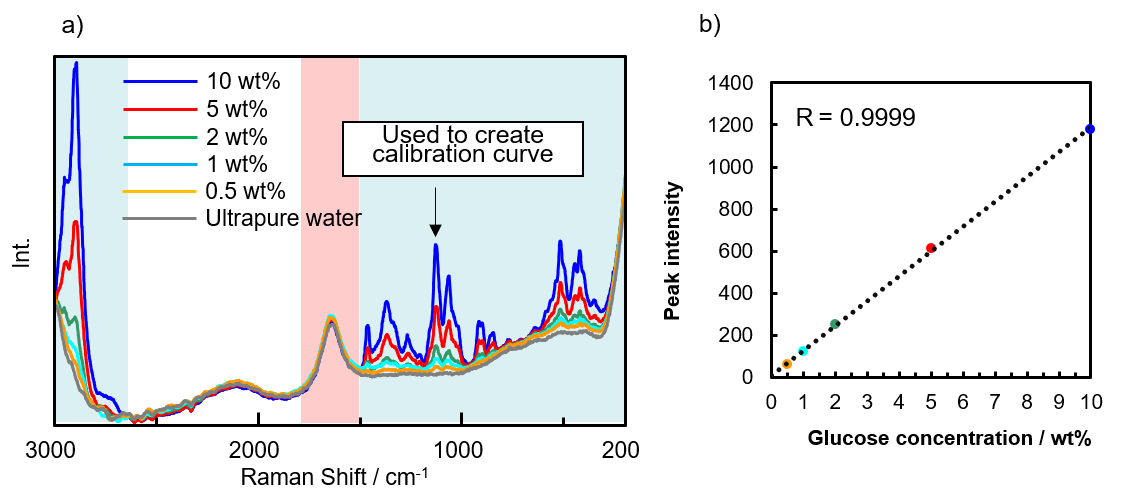
Fig. 2 a) Spectra for glucose solutions and ultrapure water, b) Calibration curve
Conclusion
In this report, we described an example of calibration curve creation for aqueous glucose solutions. Quantitative analysis of samples such as aqueous solutions of other components and inorganic salt solutions is possible using Raman spectroscopy as long as solute-derived peaks are detectable. Using the Palmtop Raman spectrometer, highly accurate quantitative measurement results can be obtained simply by placing a cuvette in the Cuvette holder.
References
1) H. Ikarashi, Y. Numata, H. Tanaka: The 26th Annual Conference of the Japan Society of Material Cycles and Waste Management, 26, 235 (2015). DOI: 10.14912/jsmcwm.26.0_235