Introduction
It is well known that basically, SFC with a column of high polarity material shows the same retention action as normal phase chromatography, and therefore it is believed to be difficult to separate aqueous high polarity components. However, if a little volatile acid, base, or salt is added to modifier solvent (alcohol or etc.), the shape of polar component’s peak can be improved, and components with long retention time can be eluted with appropriate retention time.
In this experiment, basic drugs were separated using 2-Ethylpyridine as column, Ammonium acetate in Methanol as modifier solvent.

LC-4000 SFC System
Experimental
Measurement conditions
Column: 2-Ethylpyridine 60A (4.6 mmID x 250 mmL, 5 µm, Princeton Chromatography Inc.)
CO2 Flow rate: 3.0 mL/min
Modifier: 20 mM Ammonium acetate in Methanol
Modifier gradient: 0 min (0.2 mL/min)→6 min (0.2 mL/min)→13 min (1.0 mL/min)→ 18 min (1.0 mL/min)→18.05 min (0.2 mL/min) 1 cycle: 30 min
Column temp.: 40ºC
Back Pressure: 15 MPa
Wavelength: 200-400 nm
Injection volume: 5 µL
Standard sample: Mixture 0.1 mg/mL each
Results
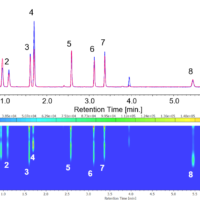
Chromatogram and contour plot of standard mixture of basic drugs (wavelength: at 220 nm) are shown in Fig.1. Polar components such as Berberine and Maleic were also eluted.
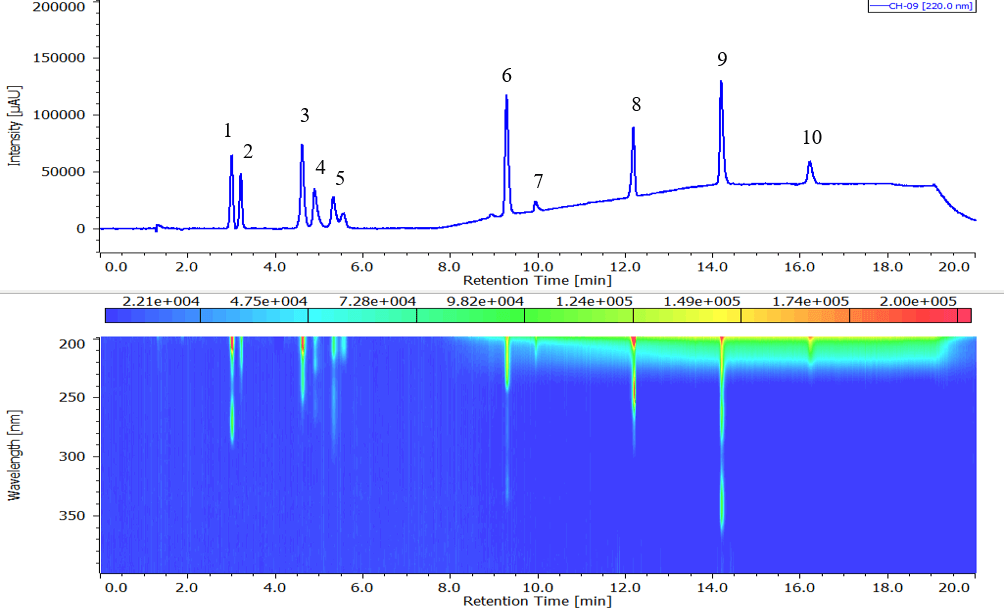
Fig. 1. Chromatogram of standard mixture of basic drugs
1: Caffeine, 2: Hexobarbital, 3: Amitriptyline, 4: Chlorpheniramine, 5: Imipramine, 6: Quinine, 7: Atropine, 8: Acetaminophen, 9: Berberine, 10: Maleic acid