Introduction
What is FRET
Fluorescence resonance energy transfer (or Förster resonance energy transfer), commonly referred to by the acronym FRET, is a mechanism by which energy transfer occurs directly between fluorescent molecules or chromophores without optical emission. The light energy absorbed by one atomic group (donor) is transferred to another atomic group (acceptor) by electron resonance. This causes the acceptor to emit fluorescence at a different wavelength to that for the donor chromophore, or to emit fluorescence at a lower intensity than that normally emitted by the donor due to quenching.
The efficiency of this energy transfer process is inversely related to the sixth power of the distance between the donor and acceptor. Therefore, in general, the donor-acceptor distance should be within 1 to 10 nm because the efficiency of FRET decreases rapidly with increasing distance.
Applications of FRET Techniques
As described above, FRET can only occur when energy donors and acceptors are in close proximity to each other, so the presence or absence of FRET is an indicator of the separation between atomic groups such as molecules and chromophores. For instance, when an additive is mixed with target proteins or DNA, the occurrence of FRET suggests that the additive is bound to the target molecules.
In fact, many protein studies have been conducted using FRET. For example, the technique has been used to investigate the affinity between bovine serum albumin (BSA) and aromatic compounds.1-4)
The half maximal inhibitory concentration (IC50) is often used to evaluate the affinity between a compound and a target using FRET.5-7) IC50 represents the concentration of a compound that is required to inhibit half the functionality of its target, and the smaller this value, the more bioactive the compound is at low concentrations.
This article shows a FRET analysis of the interaction between BSA and methylene blue (MB) obtained using a spectrofluorometer and a microplate reader using the procedure reported in Ref. 1, and a comparison of the calculated IC50 values. An automatic microplate reader has the advantage of reducing the required sample volume compared to a regular four-sided transparent rectangular cuvette, allowing high-throughput measurements of multiple samples.
Experimental
Sample
A)BSA buffer solution (pH 7.0) Final concentration 10 µM
B)MB aqueous solution Final concentration 0 µM-500 µM
System
Instrument: FP-8350 Spectrofluorometer
Accessory: FMP-125 Automatic Microplate Reader

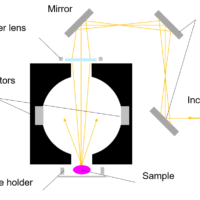
Fig. 1 FP‐8350 + FMP-125
Parameters
Ex bandwidth: 5 nm
Em bandwidth : 5 nm
Ex wavelength: 280 nm
Measurement range: 310 nm-700 nm
Response: 0.5 sec
Data interval: 0.2 nm
Scan speed: 500 nm/min
Number of wells: 96
Final volume: 300 mL
Keywords
FRET, BSA, multiple samples, trace amount, automatic microplate reader
Results
Fluorescence spectra
In the wells of a microplate, a MB solution was added to a BSA solution to obtain specific concentrations, and fluorescence spectra were then measured. The results show that the higher the MB concentration, the lower the BSA-derived fluorescence intensity (Figure 2). This indicates that the energy generated by the excitation of tryptophan residues in BSA is transferred to MB by FRET, and the tryptophan fluorescence that would otherwise be generated is quenched.
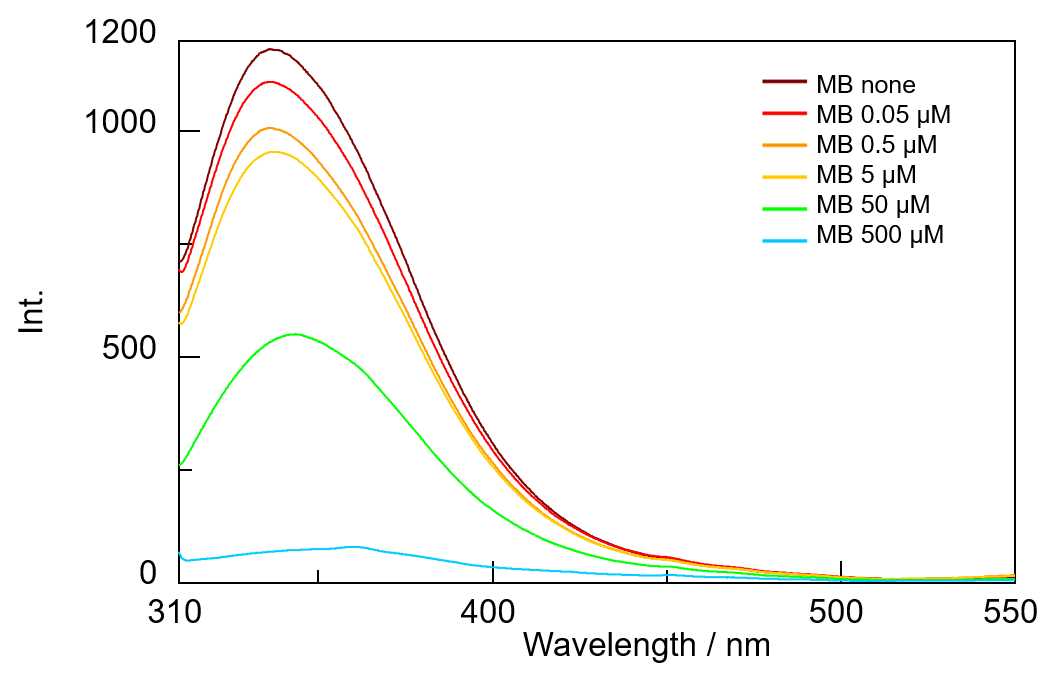
Fig. 2 Fluorescence spectra of BSA+MB with different concentrations
Dose-response curve
Figure 3 shows the relationship between the fluorescence intensity in Figure 2 at a wavelength of 336 nm and the concentration of MB. This plot is called a dose-response curve. It represents the relationship between the amount of inhibition of the target enzyme, or the amount of binding to receptors, and the concentration of the inhibiting substance. This graph is used to express the ineffective, effective, or intoxicating dose of a drug.
The IC50 value for MB was found to be approximately 30 mM. Since this is the same order of magnitude as the value reported in the literature,1) the measurement results obtained using the JASCO microplate reader appear to be reasonable.
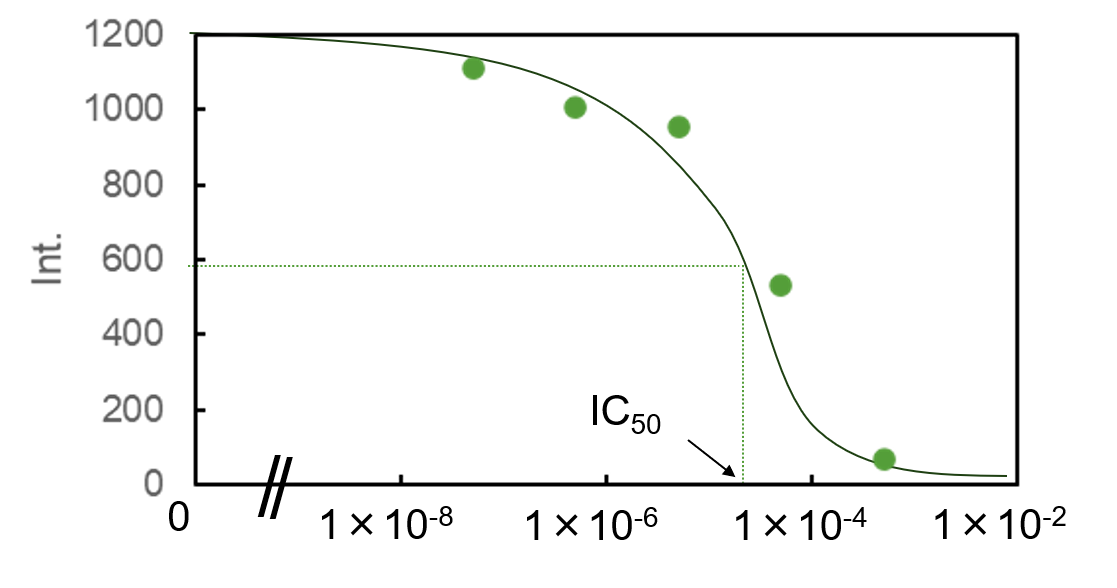
Fig. 3 Plot of fluorescence intensity at 336 nm against MB concentration
Conclusion
FRET occurring in a mixed BSA/MB solution was detected using an FMP-125 Automatic Microplate Reader. Since 50 mM and 500 mM MB solutions are dark blue in color, this can cause problems associated with internal shielding if a standard rectangular cuvette is used. Since the microplate reader is designed for sample irradiation with excitation light from above, and also for fluorescence detection from above, it is less affected by this internal shielding effect. Additionally, the microplate reader is convenient for measuring trace amounts of sample and large numbers of samples, making it effective for FRET measurements such as those described in this example.
References
1.Y.-J. Hu, Y. Liu, R.-M. Zhao, J.-X. Dong, S.-S. Qu: J. Photochem. Photobiol., A, 179, 324 (2006). DOI: 10.1016/j.jphotochem.2005.08.037
2.D. Pan, B. Jana, J. Ganguly: J. Appl. Polym. Sci., 139, 52236 (2022). DOI: 10.1002/app.52236
3.S. Bhuin, S. Halder, S. K. Saha, M. Chakravarty: RSC Adv., 11, 1679 (2021). DOI: 10.1039/d0ra09580j
4.J. Jayabharathi, V. Thanikachalam, K. Jayamoorthy: J. Photochem. Photobiol., B,, 115, 85 (2012). DOI: 10.1016/j.jphotobiol.2012.06.014
5.D. Patnaik, J. Xian, M.-A. Glicksman, G.-D. Cuny, R.-L. Stein, J.-M.-G. Higgins: J. Biomol. Screening, 13, 1025 (2008). DOI: 10.1177/1087057108326081
6.I. Boichenko, S. Deiss, K. Bär, M.-D. Hartmann, B.-H. Alvarez: J. Med. Chem., 59, 770 (2016). DOI: 10.1021/acs.jmedchem.5b01735
7.S. Lee, D.-A. Abed, L.-J. Beamer, L. Hu: SLAS Discovery, 26, 100 (2021). DOI: 10.1177/2472555220935816