Introduction
The biogenic mineral of the gallstone, seashell, coral and so on is in many cases made of calcium carbonate. There are two kinds of crystal polymorphism such as calcite and aragonite due to the difference of the crystal structure. The aragonite is more thermally unstable than the calcite at ordinary temperature and pressure, and when heated, the aragonite changes the phase to the calcite. The crystal formation depends on the organism species. On the other hand, in some cases, the calcite and aragonite are formed contiguously in the same species.
According to the recent research, if the Mg2+ is taken in the calcite crystal as an impurity, the solubility of the calcite is increased, resulting the inhibition of the crystal growth. On the other hand, the aragonite cannot take in the Mg2+ in the crystal lattice due to the ionic radius, therefore the solubility is not changed. There are a lot of Mg2+ in the sea water these days, so the aragonite is formed more in the sea due to the relation of the crystal formation in the sea and kinetic aspect of the dissolution.

Fig.1 Observation image of 5x Objective lens
In Raman spectroscopy, it is easier to measure in the low wavelength range, and it is possible to identify the polymorphism by peak pattern of the lattice vibration of the crystal appearing in the low wavenumber range. This time, the coral larva was transferred to the laboratory, then it was grown under the condition of some sea ingredient composition with adjusted the concentration of the each ions for easy growth of the calcite skeleton. The process of the skeletal formation of the calcite was measured by using the mapping measurement with Raman spectroscopy.
Experimental
Instrument: NRS-5500
Objective lens: 20x
Interval: 10 µm
Measurement points: 31 x 31, total 300 x 300 µm
Ex wavelength: 532 nm
Laser power at sample point: about 10 mW
Measurement time: about 1 hour
Sample: 1 seed coral
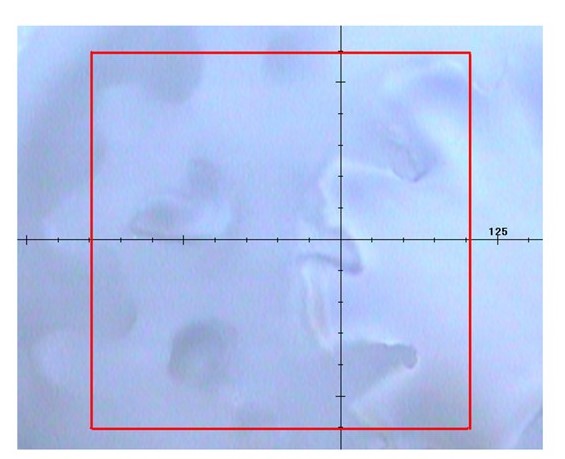
Fig. 2 Observation image of 20x objective lens (300 x 300 um in the red square)
Results
The peak due to the symmetrical stretch vibration specific to carbonate ion was observed at 1090 cm-1 from the result of the mapping measurement. In the wavenumber region below 300 cm-1, the difference of the peak patterns due to the lattice vibration depending on the crystal configuration difference between the calcite and aragonite was seen as shown in Fig. 3.
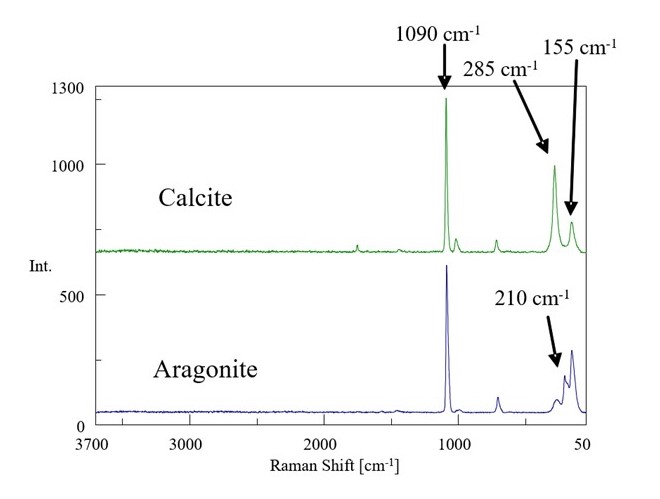
Fig. 3 Raman spectrum of calcite and aragonite
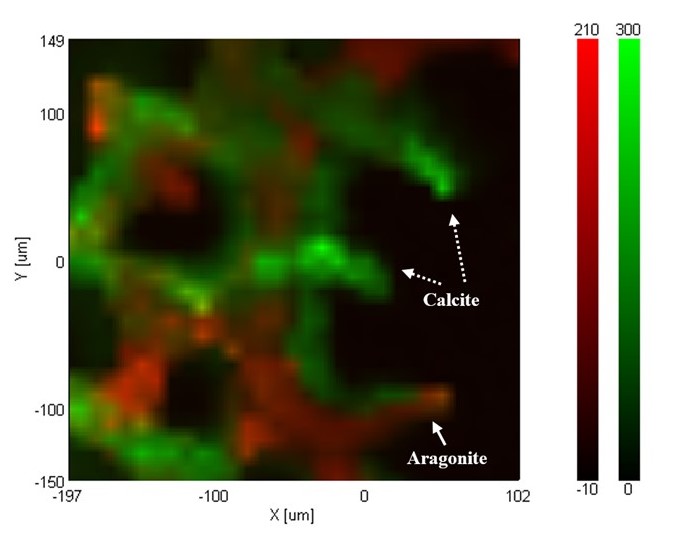
Fig.4 Color-coded plot of peak height of calcite (Green) Color-coded plot of peak height of aragonite (Red)
References
The sample is provided from Dr. Tamotsu Oomori, Dr. Hiroyuki Fujimura, and Dr. Tomihiko Highchi, University of the Ryukyus, Chemistry, Biology and Marine Science.