Introduction
Singlet oxygen is highly active and destroys biological molecules. Recently, its use for destroying cancer cells or bacteria has been researched. Herein, we describe measurements of phosphorescence at 1270 nm emitted by singlet oxygen using a JASCO near-infrared (NIR) FP-8700 spectrofluorometer with Eosin Y as a photosensitizer.
Mechanism for singlet oxygen formation
Normally, oxygen molecules are in a triplet ground state, but by providing energy they can be excited to produce singlet oxygen. However, this transition is forbidden and does not proceed directly; for this reason, a photosensitizer is used. Optical irradiation is used to excite the photosensitizer from its singlet ground state to a singlet excited state. It then changes to a triplet excited state by intersystem crossing. If the energy difference between this triplet state and the singlet ground state for the photosensitizer is almost the same as that between the singlet excited state and the triplet ground state for oxygen, energy transfer occurs. As the triplet excited photosensitizer loses its energy, singlet oxygen is produced.
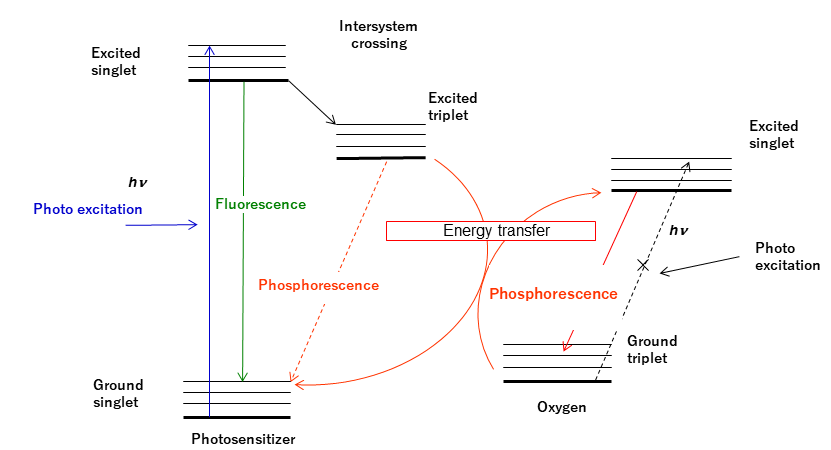
Figure 1. Mechanism for singlet oxygen generation
Experimental
System
FP-8700: Spectrofluorometer
Samples
5-ppm EosinY (ethanol solution)
Conditions
Measurement mode: Emission
Excitation range: 300-700 nm
Excitation step: 5 nm
Emission range: 1200-1350 nm/min
Data interval: 2 nm
Keywords
Singlet oxygen, NIR fluorescence, phosphorescence, photosensitizer
Results
EEM (Excitation Emission Matrix) of ethanol solution of EosinY is shown below. The phosphorescence of singlet oxygen around 1270 nm could be detected when excitation wavelength range is 470-560 nm.
Measurement of EEM of singlet oxygen reveals the suitable excitation wavelength as well as that singlet oxygen exists or not. Consequently, JASCO FP-8700 works well for research of singlet oxygen.
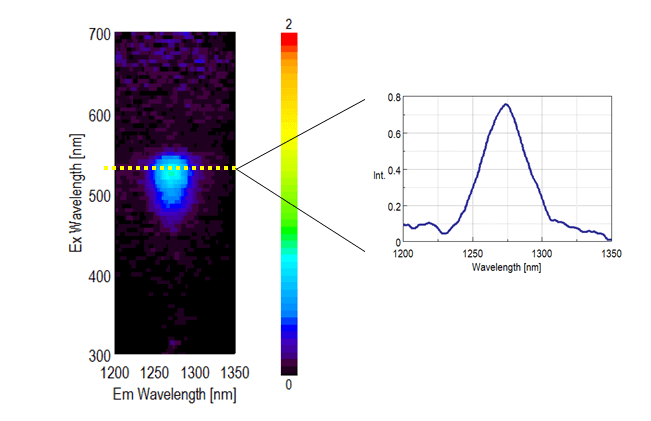
Figure 2. EEM and emission spectra at excitation wavelength of 525 nm for singlet oxygen
References
Acknowledgments
We would like to thank Prof. Takumi Harada at Oita university for providing the sample.