Introduction
In recent years, nail products using a photo-curable resin, referred to as gel nails, have been widely used. Gel nails adhere to real nails due to a photo-polymerization reaction that cures colored synthetic resins under ultraviolet or visible light irradiation. Gel nails generally have a base coat to ensure adhesion to the nail surface, a color coat in which dyes or pigments are dispersed, and a top coat to increase the strength and to support decorations. There are also one-step types that do not require pre- or post-treatment of fingernails. For both types of gel, the photo-initiator is radicalized by photo-irradiation, and polymerized by a radical chain reaction with functional groups of the monomer or oligomer (Figure 1).
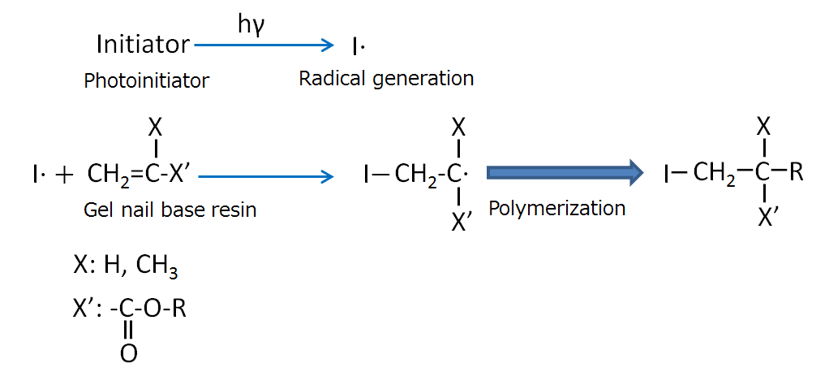
Figure 1 Reaction mechanism for photo-curing resin used for gel nails
If the target reaction mechanism does not proceed properly, uncured gel can cause allergies, so it is important to assess the photo-polymerization reaction process in resin development. In order to evaluate the reaction efficiency in resin development, we investigated the photopolymerization reaction process using an infrared spectrophotometer for one-step gel nail products, which are acrylate-based photo-curing materials.
Experimental
A sample (commercial one-step gel nail) was placed on an ATR prism, and time-dependent spectra were measured using the ATR method during the curing process under photo-irradiation. Photo-irradiation of the upper sample surface (opposite side to the prism) using a pen-type light was initiated 15 seconds after the start of measurement.
Measurement parameters
Method: ATR
Resolution: 4 cm-1
Detector: DLATGS
Crystal: Diamond
Measurement time: 180 s
Accumulations: 1

FTIR spectrometer with ATR accessory (FT/IR-4600 + ATR PRO ONE)
Keywords
Gel nails, photo-curable resins
Results
Curing of gel nails can be monitored by the decrease in the number of unsaturated carbon double bonds (C=C) that are abundant in oligomers and monomers, and by changes in the ester groups in the polymer after curing. Figure 2 shows a three-dimensional spectrum, and Figure 3 shows an IR spectrum before and after photo-irradiation.
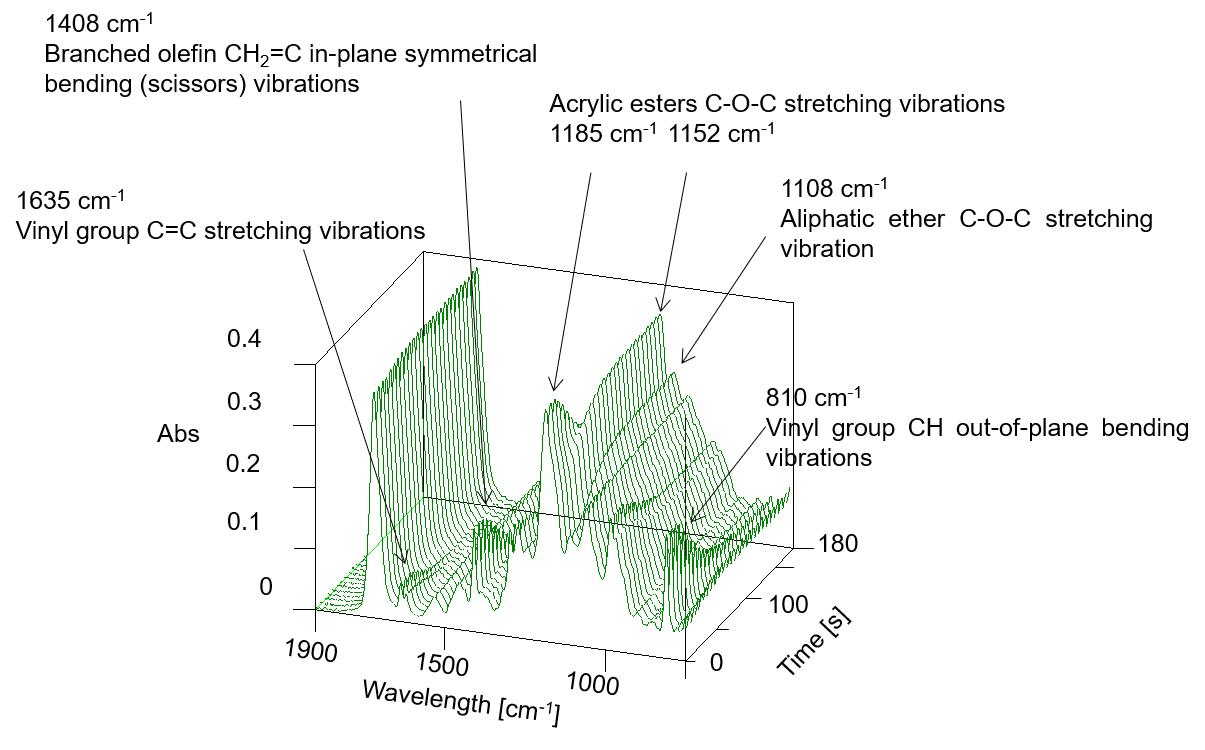
Figure 2 Three-dimensional spectrum
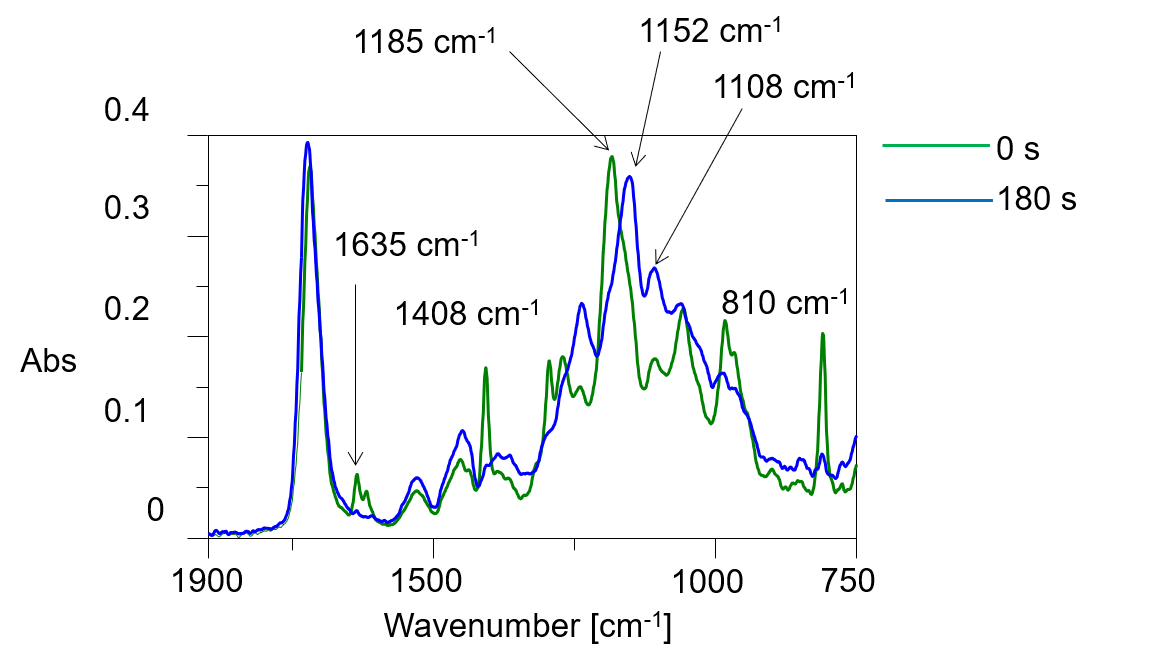
Figure 3 IR spectra before and after photo-irradiation
In these figures, it is clear that the intensity of the absorption bands assigned to C=C stretching vibrations near 1635 cm-1 and the bands due to C=CH in-plane symmetrical bending vibrations near 1408 cm-1 decrease with photocuring. In addition, the absorption band assigned to out-of-plane vibrations of C=CH near 810 cm-1 also decreases in intensity with increasing reaction time. Furthermore, the absorption band assigned to the -C-O-C- bond near 1100 cm-1 changes due to polymerization. Figure 4 shows the time variation of the intensity of the different absorption peaks. It can be seen that the peak intensity changes from about 15 seconds (immediately after light irradiation) to 90 seconds.
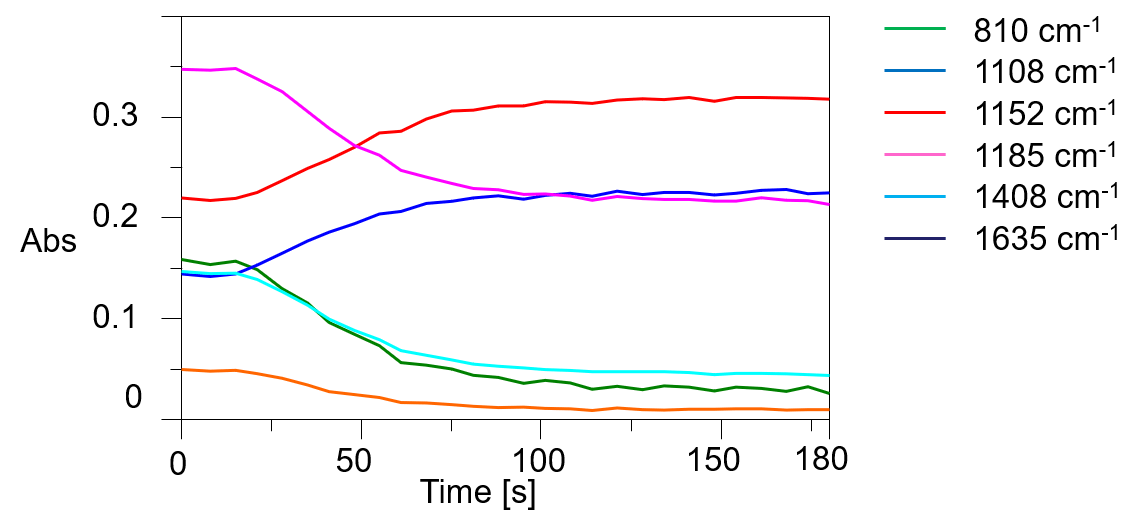
Figure 4 Time variations of peak height
Conclusion
In this experimental system, the curing light irradiated the sample on the surface opposite the prism in order to reproduce the situation when a gel nail is applied to a fingernail, and the reaction at the prism/sample interface (actual fingernail side where it is difficult for the curing light to reach) was monitored using IR spectroscopy. We believe that the reaction at the fingernail surface can be evaluated based on such measurements. If the reaction at the prism/sample interface is complete, it is considered that the reaction in the entire sample is complete, so the results can be used to evaluate uncured gel that may cause allergies. Thus, it was shown that measurement of time-dependent IR spectra using the ATR method is an effective tool for evaluating the gel nail curing process.
Please also refer to the related article, which describes an evaluation of the beauty of gel nails.