Introduction
Aldehydes are an environmental pollutant, and there is concern about polluting environmental water such as the atmosphere, lakes, reservoirs, and rivers. In Japan, aldehydes are subject to various regulations such as the Air Pollution Control Act, Offensive Odor Control Law (Ministry of the Environment), Industrial Safety and Health Act and Water Supply Act (Ministry of Health, Labour and Welfare). Above all, formaldehyde is known as one of the causative agents of sick house syndrome, and it is warned to potentially have carcinogenic effects.
Formaldehyde has become a standard compound (standard value: 0.08 mg/L) from previous monitoring according to the notification of Ordinance No. 101 of the Ministry of Health, Labour and Welfare on May 30, 2003. Acetaldehyde was also set as a compound to be examined.
In this paper, the SCIEX triple quadrupole mass spectrometer ‘Triple QuadTM 3500’ was used as a detector to analyze formaldehyde and acetaldehyde derivatized by 2,4-Dinitrophenylhydrazine (2,4-DNPH) using LC/MS/MS. It was confirmed that detection was possible down to 0.008 mg/L, which is 1/10 of the standard value of 0.08 mg/L. In addition, we also conducted an add and recovery test for tap water, which is reported below.

Experimental
[Equipment]
Pump: PU-4185-Binary
Column oven: CO-4060
Autosampler: AS-4150
Autosampler Option: TC-4000-2
Detector: SCIEX Triple Quad 3500TM
System Control: Analyst® Jasco Analyst
[HPLC Conditions]
Column: Unifinepak C18 (2.0 mmI.D. x 100 mmL, 1.9 mm)
Eluent : Acetonitrile / Water (50 / 50)
Flow rate: 0.2 mL/min
Column temp.: 40 ˚C
Injection volume: 1 mL
[MS Conditions]
Ionization: ESI, Negative
MRM 2,4-DNPH-formaldehyde Q1/Q3: 209/151
2,4-DNPH-acetoaldehyde Q1/Q3: 223/163
Standard sample: 2,4-DNPH-formaldehyde, 2,4-DNPH-acetoaldehyde
0.005 ~ 0.1 mg/L
Keywords
Formaldehyde, Acetaldehyde, Water quality standards, 2,4-DNPH, Pre-column derivatization, LC-4000, Triple QuadTM 3500, LC/MS/MS, Unifinepak C18, JASCO
Results
Fig. 1 shows the reaction formula for 2,4-DNPH precolumn derivatization, and Fig. 2 shows the aldehyde derivatization method. The aldehyde derivatization was performed using the 1/10 scale described.
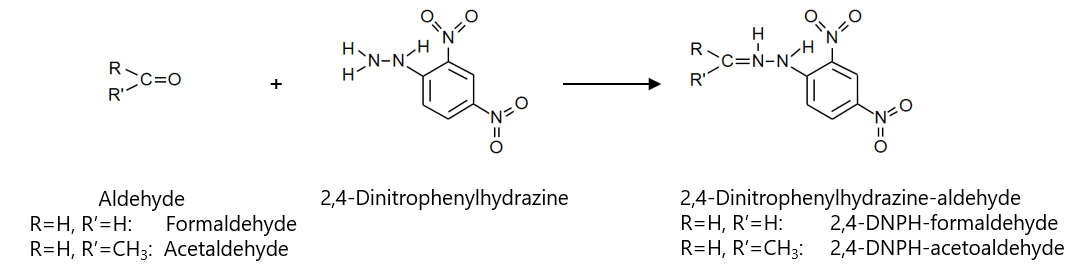
Fig. 1 reaction formula for 2,4-DNPH precolumn derivatization
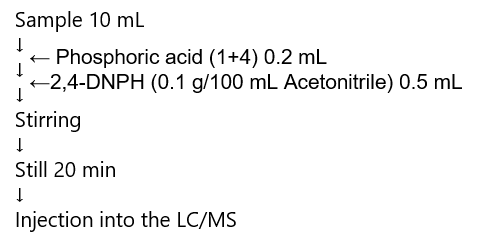
Fig. 2 Aldehyde derivatization method
Fig. 3 shows the chromatogram of the 2,4-DNPH formaldehyde and 2,4-DNPH acetaldehyde standard mixed solution.
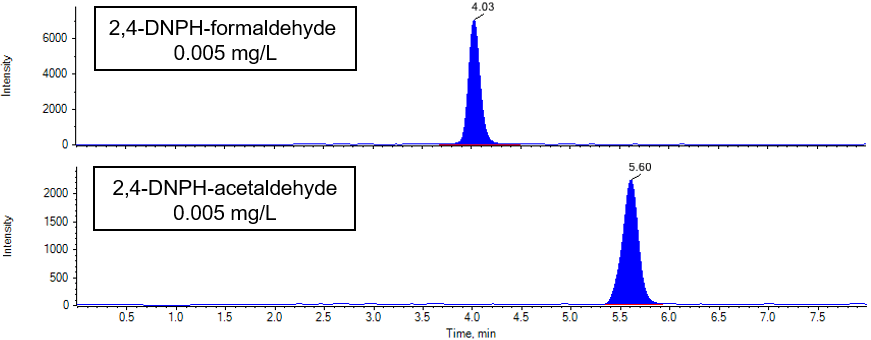
Fig. 3 Chromatogram of 2,4- DNPH formaldehyde and 2,4-DNPH acetaldehyde (0.005 mg/L)
Fig. 4 shows the calibration curve. As a result of plotting 5 concentrations of 2,4-DNPH formaldehyde and 2,4-DNPH acetaldehyde from 0.005 to 0.1 mg/L, good linearity of 0.9998 and 0.9999, respectively, was obtained.
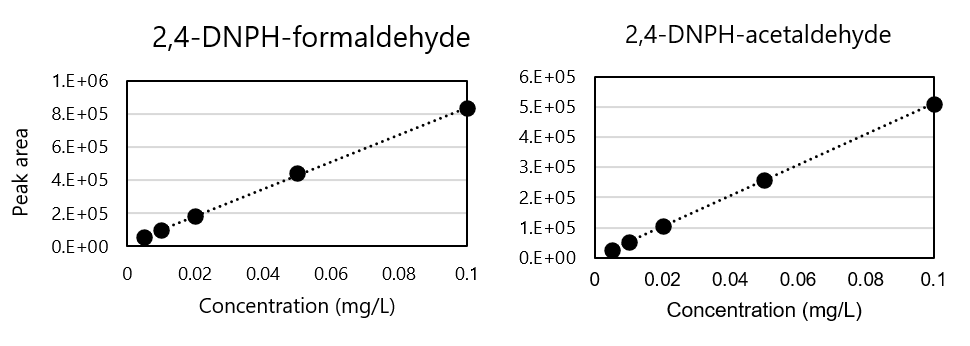
Fig. 4 Calibration curve of 2,4-DNPH formaldehyde and 2,4-DNPH acetaldehyde
Table 1 shows the reproducibility of 2,4-DNPH formaldehyde at 0.005, 0.01, and 0.1 mg/L (n=5), and Table 2 shows the reproducibility of 2,4-DNPH acetaldehyde of 0.005, 0.01, and 0.1 mg/L (n=5). Good reproducibility was obtained at 0.005 mg/L, which is less than 1/10 the standard value.
Table 1. Peak area and retention time reproducibility of 2,4-DNPH formaldehyde (n=5)
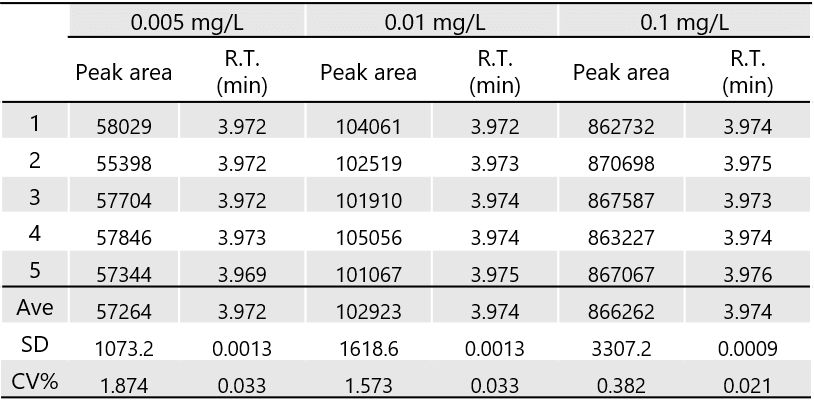
Table 2. Peak area and retention time reproducibility of 2,4-DNPH acetaldehyde (n=5)
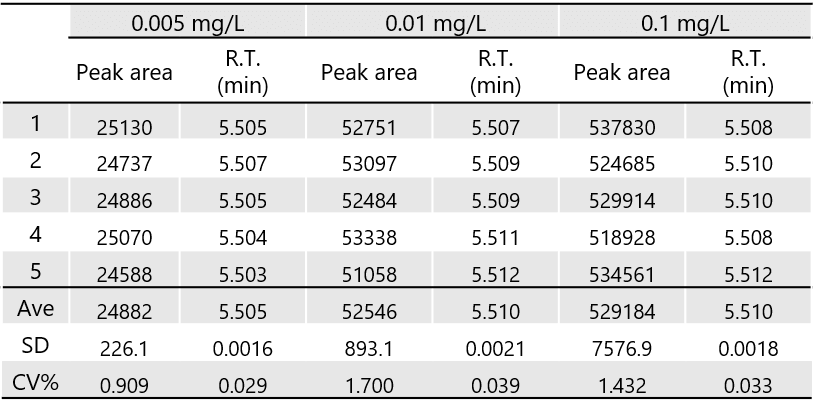
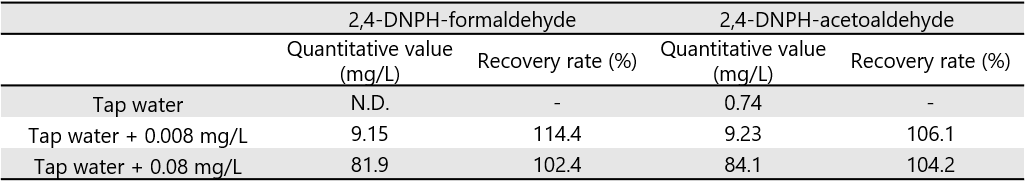
Fig. 5 and Table 3 show the results of the additive recovery measurements on tap water. The recoveries when formaldehyde and acetaldehyde were added to tap water to 0.08 mg/L and 0.008 mg/L were 102.4, 104.2% (0.08 mg/L) and 114.4, 106.1% (0.008 mg/L), respectively. Good results were obtained within 70 to 130% of the reference concentration.
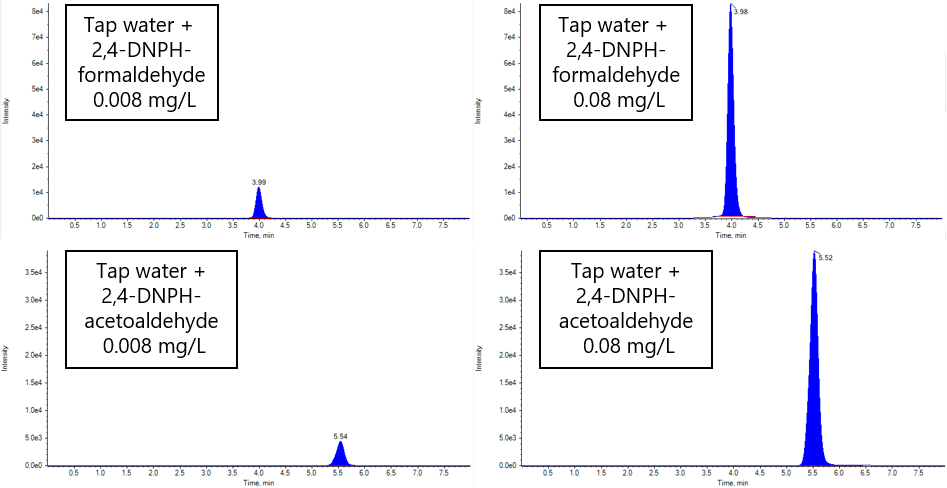
Fig. 5 Chromatogram of additive recovery test to tap water
(Left: 0.008 mg/L added, Right: 0.08 mg/L added)
Table 3 Additive recovery test results to tap water