Introduction
When used in infectious disease control, the ethanol concentration in ethanol-based antiseptics used for hand sterilization should be between 70 and 83 percent by volume (vol%). Recently, as a temporary measure against the novel coronavirus, the use of 60 vol% has been allowed when ethanol is difficult to obtain*1. When it is necessary to use high-concentration ethanol products other than general antiseptics, they must be diluted with purified water prior to use. It is important to confirm the correct ethanol concentration for the diluted product.
FTIR spectroscopy is a fast and accurate analytical technique for quantitation of ethanol concentration in liquids. This application note reports the results for the analysis of ethanol concentrations in three commercially available ethanol-based antiseptics and a beverage grade alcohol that can be used for hand sterilization.
*1 According to materials released by the Ministry of Health, Labor and Welfare, Japan
Experimental
The ATR measurement was used without any sample pretreatment. Liquid samples are measured simply by placing a droplet on the prism. The analysis time per sample is approx. 4 seconds, and the ethanol concentration is reported automatically. Spectra Manager’s “Simple Quantitation” option in the standard Spectra Measurement program allows quantitation and Pass/Fail judgment at the same time as measurement.
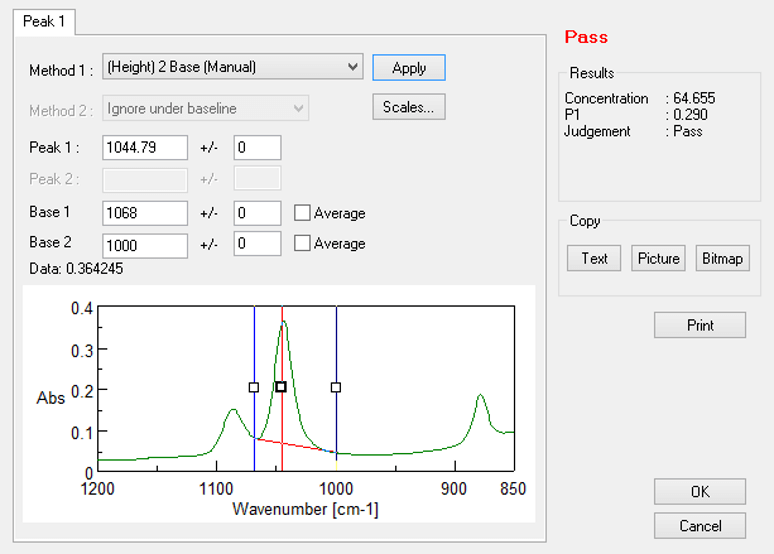
Simple Quantitation function in the Spectra Measurement program
Measurement conditions
Main unit: FT/IR-4600
Detector: DLATGS
Accessory: ATR PRO ONE
ATR crystal: Diamond
Resolution: 4 cm-1
Accumulation: 4 (Approx. 4 seconds)

FTIR spectrometer with ATR Accessory (FT/IR-4600 + ATR PRO ONE)
Results
Calibration curve
Standards from 0 to 99.5 vol% was prepared by mixing 99.5 vol% ethanol reagent with ultrapure water. The IR spectrum of 99.5 vol% ethanol is shown in Figure 1 (left). Figure 1 (right) also shows the calibration curve for the standard samples created at the peak height of 1045 cm-1 attributed to the C-O stretching vibration.
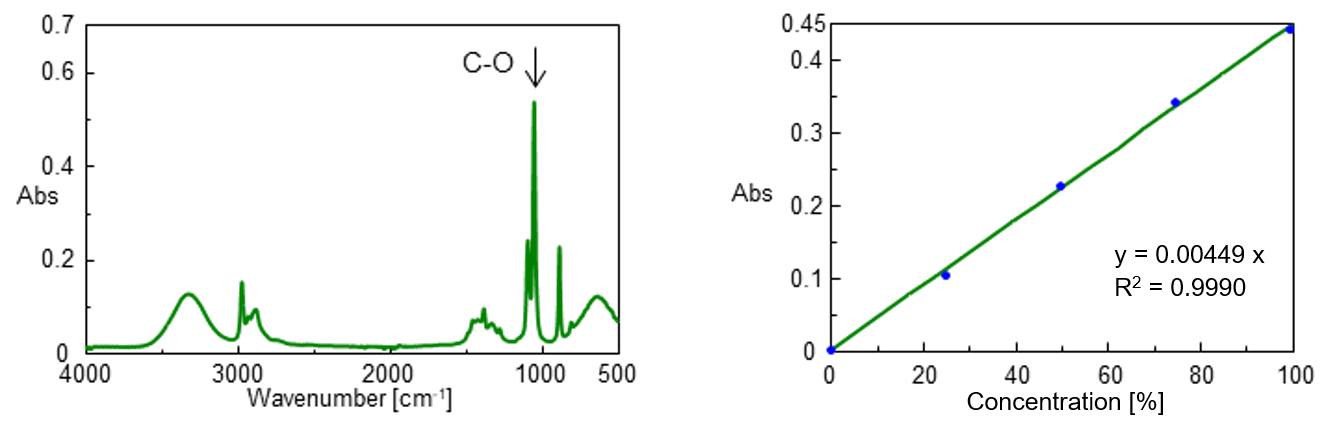
Figure 1. IR spectrum of ethanol (left) and calibration curve (right)
Quantitation result
The ethanol concentrations of five samples: three commercially available antiseptics, a high concentration beverage alcohol, and as a reference, a Chinese liquor with a high alcohol concentration were measured. The following table shows the quantitation results and pass/fail judgment results. A concentration in the range 60 to 83 vol% was designated as “pass”. It was confirmed that the commercially available antiseptics and high-concentration beverage alcohol have concentrations that can allow them to be used as antiseptic for hand sterilization.
Table 1. Ethanol concentration quantitative results
| Sample | Ethanol concentration quantitative results (vol %) | Pass/Fail |
|---|---|---|
| Ethanol-based antiseptic A | 77 | Pass |
| Ethanol-based antiseptic B | 79 | Pass |
| Ethanol-based antiseptic C | 73 | Pass |
| High concentration beverage alcohol | 65 | Pass |
| Reference: Chinese liquor | 53 | Fail |
Conclusion
FTIR can be used to quantify not only the antiseptics, but also the ethanol concentration in alcoholic beverages. Using the same methodology, it can be used for quality control in pharmaceutical and food industries.