Introduction
In recent years, there has been a growing emphasis on improving work efficiency in the field of spectroscopic analysis. To address this issue, JASCO has developed the HTCD Plus, which is a system that can automatically measure circular dichroism (CD) and absorption spectra of multiple samples. This system allows efficient and comprehensive evaluation of the effects of organic solvents on different compounds, structural differences between similar compounds, and chemical and physical properties. This report describes examples of automated measurements of magnetic circular dichroism (MCD) and absorption spectra of phthalocyanine complexes using a permanent magnet in the sample chamber of this system.
Magnetic circular dichroism (MCD) spectroscopy is a widely used method for evaluating the electronic structure and magnetic and optical properties of highly symmetrical molecules such as phthalocyanines and porphyrins, which have photophysical properties.1-3) Metal complexes with various central metal ions, axial ligands, and substituents have been synthesized, and analysis of the electronic structure,4-5) determination of the Faraday parameters and the orbital angular momentum,6-8) and identification of chemical species9-11) based on MCD and absorption spectra have been performed. This report describes the results of evaluating the effects of substituents on the calculated orbital angular momentum Lz by automatically measuring MCD and absorption spectra for zinc phthalocyanine (ZnPc) and two ZnPc derivatives with different substituents using the JASCO HTCD Plus.
Experimental
Samples
Zinc phthalocyanine (ZnPc): Abs (670 nm) = 0.103
Zinc 2,9,16, 23-tetra-tert-butyl-29H, 31H-phthalocyanine (ZnPc1): Abs (676 nm) = 0.151
Zinc 1,4,8,11,15,18,22, 25-octabutoxy-29H, 31H-phthalocyanine (ZnPc2): Abs (739 nm) = 0.104
Solvent: N, N-dimethylformamide (DMF)
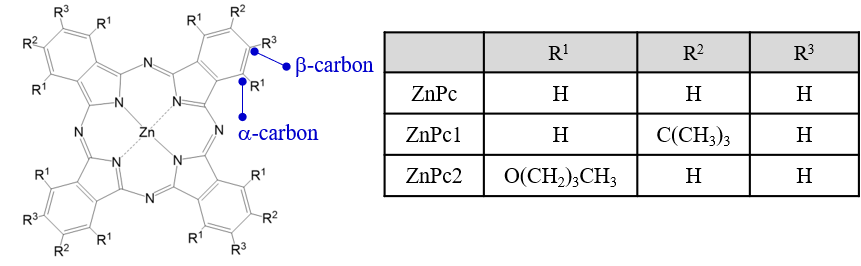
Fig. 1 Structure of ZnPc, ZnPc1 and ZnPc2
System
Instrument: J-1500 CD spectrometer
Accessories: HTCD Plus High-throughput circular dichroism system, PMCD-593 Compact permanent magnet, Flow-through cell (manufactured by Hellma)

Fig. 2 HTCD Plus, compact permanent magnet and flow cell
Operation of HTCD Plus
The HTCD Plus consists of a CD spectrometer, an autosampler, a syringe pump, a drying pump and a flow cell (Figure 3). A permanent magnet was additionally used for this measurement. The measurement procedure is as follows:
1. A sample in an individual vial or microplate well is aspirated through the nozzle of the autosampler using the syringe pump, and delivered to the flow cell.
2. The CD and MCD spectra of the sample are then measured simultaneously.
3. The sample is subsequently returned to the original vial or microplate well, or discharged into a drain bottle.
4. The flow system is flushed with a cleaning solution.
5. The flow system is then dried using the drying pump.
6. Steps 1 to 5 can be repeated 120 times for vials and 196 times for microplate wells.
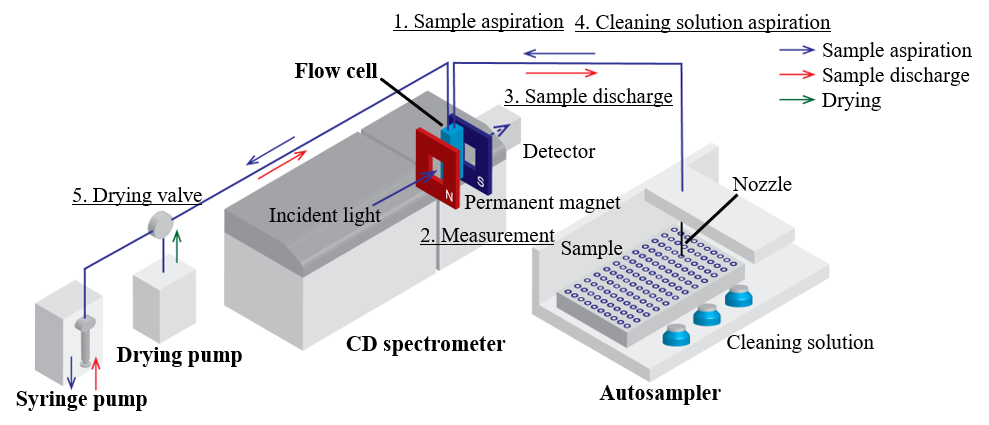
Fig. 3 Schematic diagram of HTCD Plus
Parameters
Wavelength range: 550 nm – 800 nm
Scanning speed: 50 nm/min
D.I.T.: 2 sec
Bandwidth: 2 nm
Data pitch: 0.1 nm
Cell pathlength : 5 mm
Magnetic field: 1.3 T
Keywords
Automation, increased efficiency, autosampler, substituent effect, solvent effect, circular dichroism, magnetic circular dichroism, CD, MCD
Results
Each of the three phthalocyanine complexes was dissolved in DMF, and 1 mL of each solution was then dispensed into three vials for spectrum measurement. Solvent and sample measurements were conducted alternately, and the baseline was automatically corrected using the solvent spectrum obtained immediately before the sample spectrum. After baseline correction, high-frequency noise components were removed from the spectra using a fast Fourier transformation (FFT) filter. A band deconvolution analysis was performed for the absorption and MCD spectra obtained by this procedure, and the Faraday terms A1 and B0, the dipole strength D0 and the orbital angular momentum Lz were calculatedNote). Figure 4 shows the band-resolved results for the MCD and absorption spectra obtained in the first of three repeat measurements for each sample.
Note: For details about the band deconvolution analysis, the Faraday terms and the strength of the dipole moment, refer to “CD Application Note 180-CD-0047”.
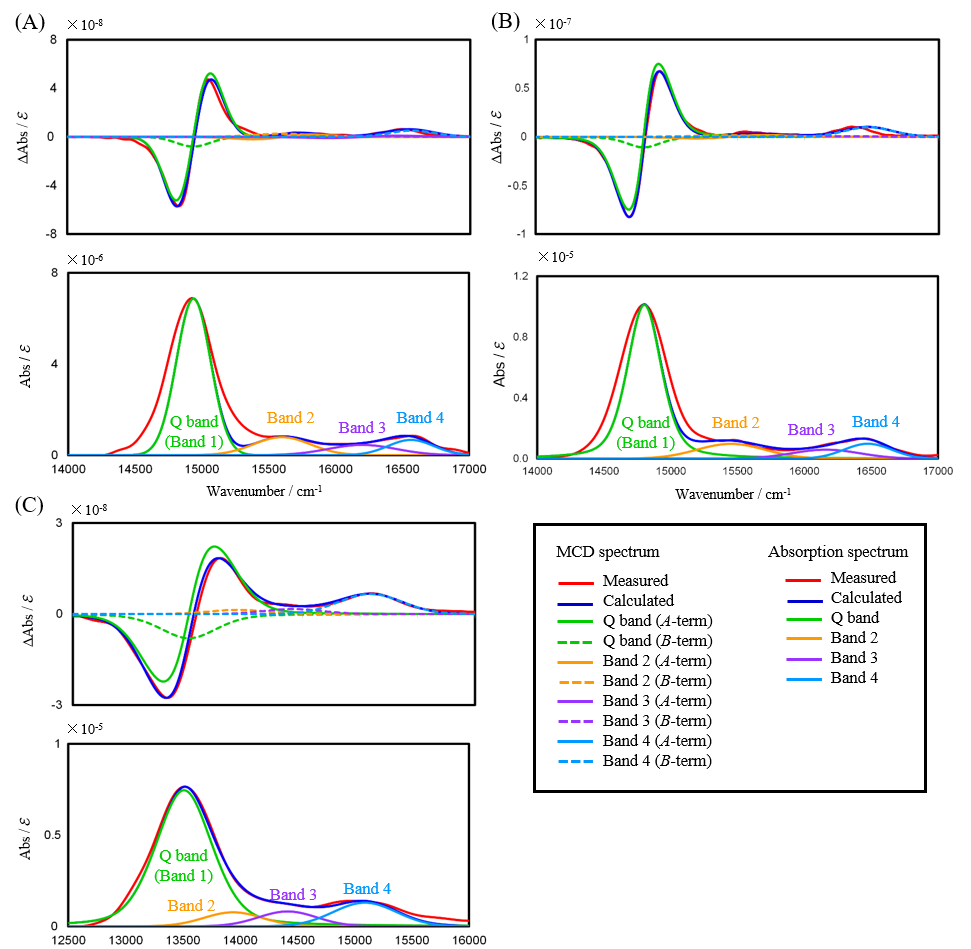
Fig. 4 Band deconvolution analysis results for MCD and absorption spectra
(A) ZnPc, (B) ZnPc1, (C) ZnPc2
A strong sharp Q band (Band 1) characteristic of monomeric phthalocyanine complexes is seen in the absorption spectra of all samples. The MCD spectrum in the Q-band region for each sample exhibits a first-derivative shape, consisting of a major Faraday A-term component and a minor B-term component. Therefore, in this analysis, after decomposing the absorption spectrum into four bands, each band in the MCD spectrum was decomposed into Faraday A and B terms.
Table 1 lists the A1∕D0 ratio for the Q band obtained by the band deconvolution analysis, together with the average value (Ave), the standard deviation (SD) and the coefficient of variation (CV).
Table 1 Reproducibility of calculation results for orbital angular momentum
| ZnPc | ZnPc1 | ZnPc2 | |
| 1 | 2.6 | 2.7 | 2.1 |
| 2 | 2.7 | 2.8 | 2.1 |
| 3 | 2.7 | 2.7 | 2.1 |
| Ave | 2.67 | 2.73 | 2.1 |
| SD | 0.0577 | 0.0577 | 0 |
| CV / % | 2.2 | 2.1 | 0 |
Here, A1∕D0 represents the absolute value of the orbital angular momentum Lz. The coefficient of variation for Lz over three measurements was extremely small, at less than 2.5%, indicating that this analysis method using the HTCD Plus provides high measurement and analysis reproducibility. Furthermore, the Lz values for ZnPc and ZnPc1 were very similar. This is thought to be due to the rigid planarity of the phthalocyanine skeleton. On the other hand, a smaller Lz value was obtained for ZnPc2, which is likely due to the steric effect caused by the butoxy group bonded to the a-carbon (Figure 1), which distorts the phthalocyanine skeleton and reduces the molecular symmetry.12) These results demonstrate that the HTCD Plus equipped with a permanent magnet can effectively evaluate the magnetic properties of phthalocyanine complexes with various substituents with good reproducibility.
Conclusion
MCD and absorption spectra were measured for zinc phthalocyanine derivatives with different substituents using the JASCO HTCD Plus and a permanent magnet, and the effect of substituents on the molecular orbital angular momentum was evaluated. The HTCD Plus is not only useful for evaluating substituent effects as described here, but also for efficiently and comprehensively evaluating solvent effects for different compounds, and differences in the structure, optical properties, and physical properties of similar compounds.
References
1)K. Kobayashi, K. Nakai: Chem. Commun., 40, 4077-4092 (2007). DOI:10.1039/b704991a
2)MGI. Galinato, EP. Brocious, FP. Paulat, S. Martin, J. Skodack, JB. Harland, N. Lehnert: Inorg. Chem., 58, 2144-2162 (2020). DOI: 10.1021/acs.inorgchem.9b02599
3)DE. Nevonen, GT. Rohde, VN. Nemykin: Inorg. Chem., 58, 14120-14135 (2019). DOI: 10.1021/acs.inorgchem.9b02138
4)S. Ghidinelli, S. Abbate, E. Santoro, S. Belviso, G. Longhi: J. Phys. Chem. B, 125, 264-280 (2021). DOI: 10.1021/acs.jpcb.0c09277
5)V. Andrushchenko, D. Padula, E. Zhivotova, S. Yamamoto, P. Bour: Chirality, 26, 655-662 (2014). DOI: 10.1002/chir.22365
6)J. Mack, MJ. Stillman, N. Kobayashi: Coord. Chem. Rev., 251, 429-453 (2007). DOI: 10.1016/j.ccr.2006.05.011
7)MJ. Stillman, AJ. Thomson: J. Chem. Soc., Faraday Trans. 2, 70, 805-814 (1973). DOI: 10.1039/f29747000805
8)A. Kaito, T. Nozawa, T. Yamamoto, M. Hatano, Y. Orii: Chem. Phys., Lett., 52, 154-160 (1977). DOI: 10.1016/0009-2614(77)85142-7
9)A. Ceulemans, W. Oldenhof, C. Gorller-Walrand, LG. Vanquickenborne: J. Am. Chem. Soc., 108, 1155-1163 (1986). DOI: 10.1021/ja00266a007
10)WR. Browett, MJ. Stillman: Inorganica Chim. Acta, 49, 69-77 (1981). DOI: 10.1016/S0020-1693(00)90460-2
11)JP. Collman, F. Basolo, E. Bunnenberg, TJ. Collins, JH. Dawson, PE Jr. Ellis, ML. Marrocco, A. Moscowits, JL. Sessler, T. Szymanski: J. Am. Chem. Soc., 103, 5636-5648 (1981). DOI: 10.1021/ja00409a003
12)JD Wang, JL Huang, JW Cai, NS Chen: Chinese J. Struct. Chem., 21, 617-620 (2002).