Introduction
This article shows the measurement result that gradient elution with PDA detector could separate 9 active ingredients (Acetaminophen, Anhydrous caffeine, Dihydrocodeine phosphate, dl-Methylephedrine Hydrochloride, Ethenzamide, chlorpheniramine maleate, Noscapine, Glycyrrhizic acid, and Ibuprofen), which have medicinal properties. In addition, it also shows the measurement results of two kinds of cold medicines.
LC-4000 HPLC system
Experimental
<Experimental conditions>
Column: Develosil ODS-HG-5 (4.6 mmID x 250 mmL, 5 µm)
Eluent A: 10 mM Sodium phosphate buffer – 100 mM Sodiuim perchlorate (pH 2.5, adjusted with phosphoric acid)
Eluent B: Acetonitrile
Gradient condition: (A/B), 0 min (90/10) -> 4.0 min (75/25) -> 6.0 min (75/25) -> 12.0 min (60/40) -> 18.0 min (20/80) -> 23.00 min (20/80) -> 23.1 min (90/10) 1 cycle: 35.0 min
Flow rate: 1.0 mL/min
Column temp.: 40ºC
Wavelength: 200-400 nm
Injection volume: 10 µL
Standard sample: 9 pharmaceutical ingredients 100 µg/mL each in 50% ethanol, except dihydrocodeine phosphate: 50 µg/mL
Structural formula of components of medicine are showed in Figure 1.
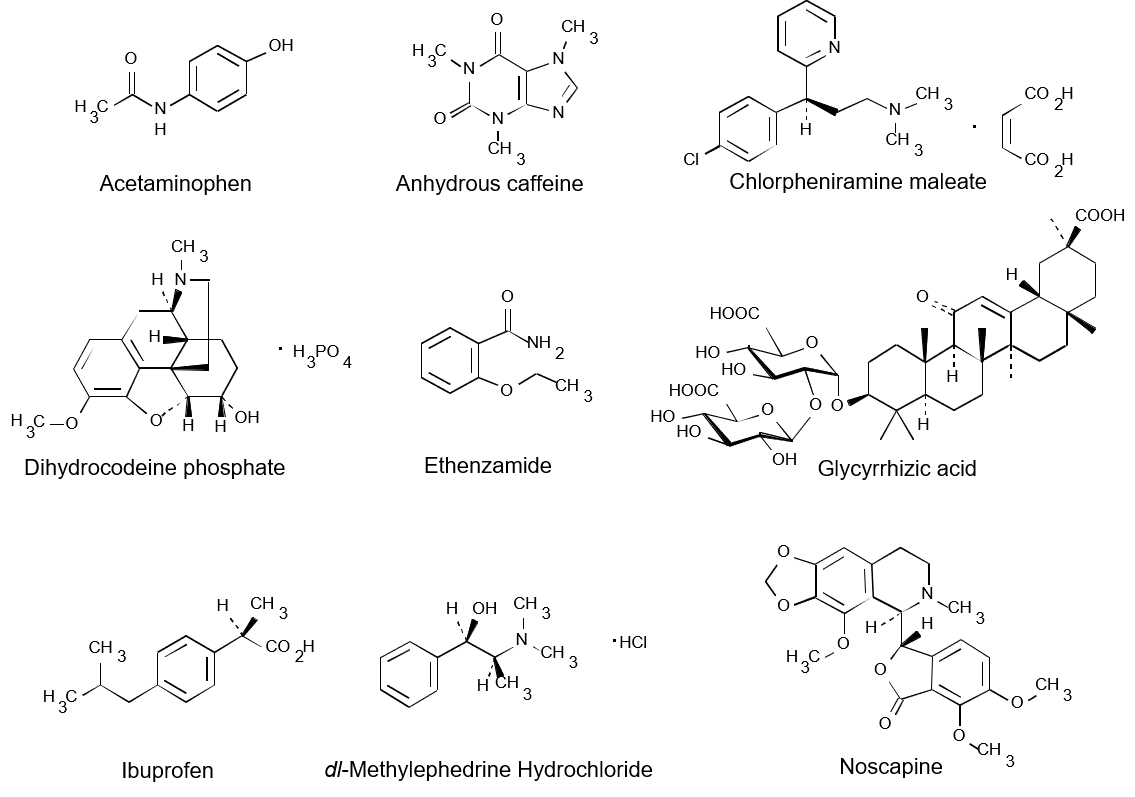
Figure 1. Structural formula of components of medicine
Keywords
OTC drug (over-the-counter drug), C18 Column, PDA Detector
Results
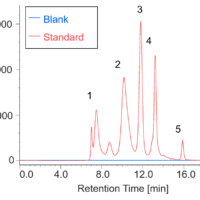
Chromatogram and contour plot of medicine’s standard mixture (9 components) are showed on Figure 2. Separation was completed within 22 minutes with good results. On peak spectra of medicine’s standard mixture (9 components) are also shown in Figure 3.
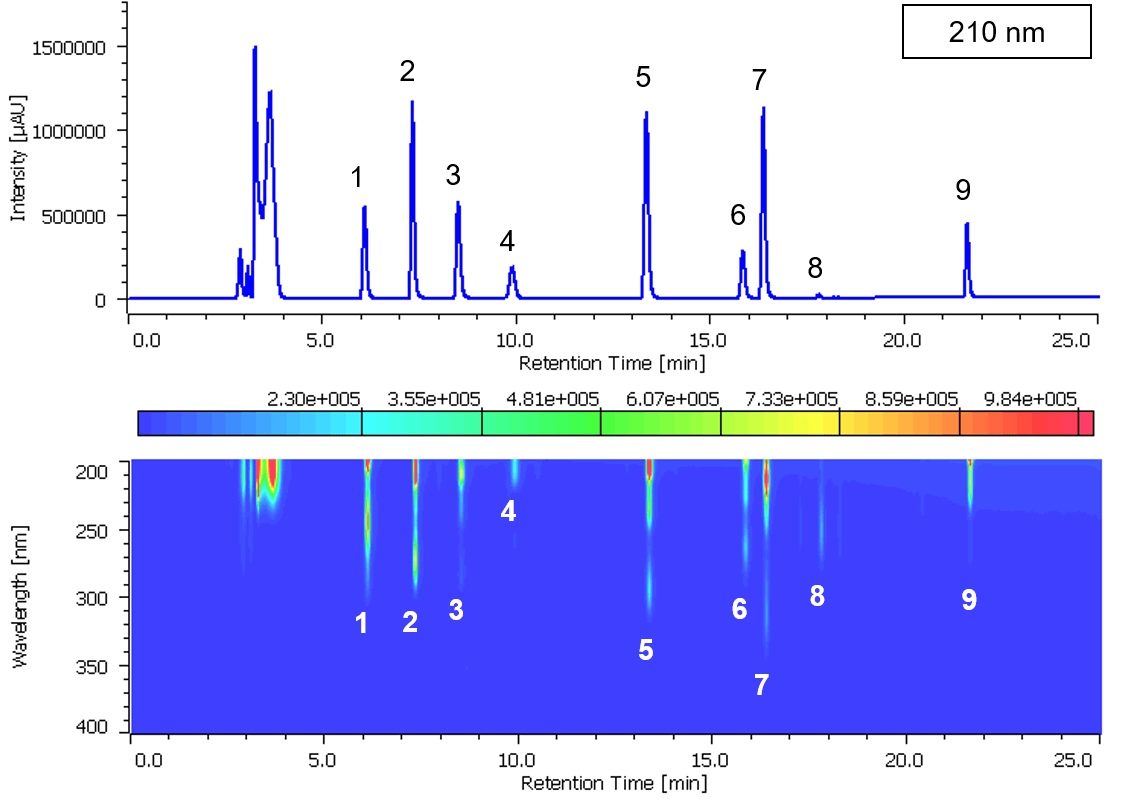
Figure 2. Chromatogram and contour plot of medicine’s standard mixture (9 components, 1: Acetaminophen, 2: Anhydrous caffeine, 3: Dihydrocodeine phosphate, 4: dl-Methylephedrine hydrochloride, 5: Ethenzamide, 6: Chlorpheniramine maleate, 7: Noscapine, 8: Glycyrrhizic acid, 9: Ibuprofen)
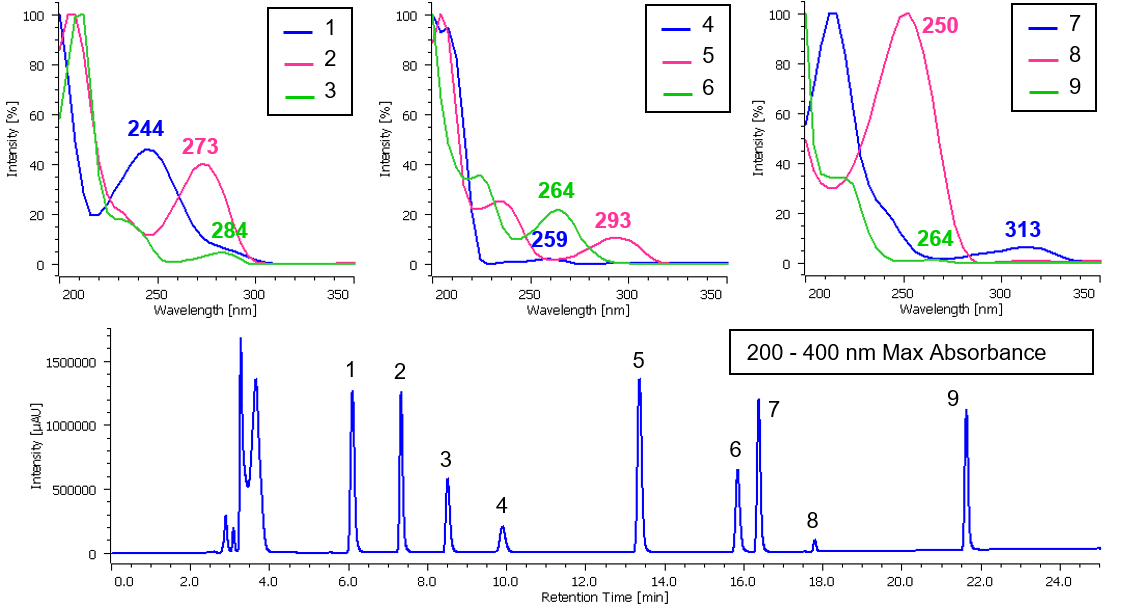
Figure 3. On peak spectrum of medicine’s standard mixture (9 components, 1: Acetaminophen, 2: Anhydrous caffeine, 3: Dihydrocodeine phosphate, 4: dl-Methylephedrine hydrochloride, 5: Ethenzamide, 6: Chlorpheniramine maleate, 7: Noscapine, 8: Glycyrrhizic acid, 9: Ibuprofen)
Procedure for sample preparation of OTC drug for cold is shown in Figure 4.
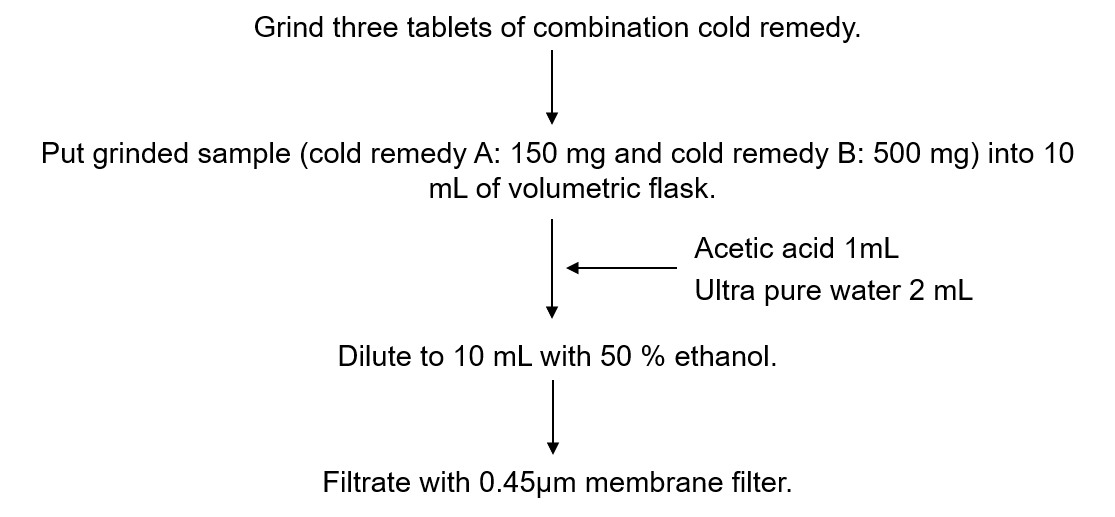
Figure 4. Procedure for sample preparation of combination cold remedy
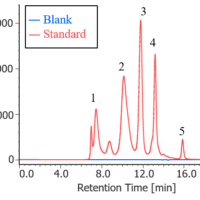
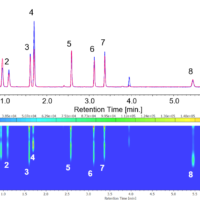
Chromatogram and contour plot of OTC drug for cold (A) are showed on Figure 5.
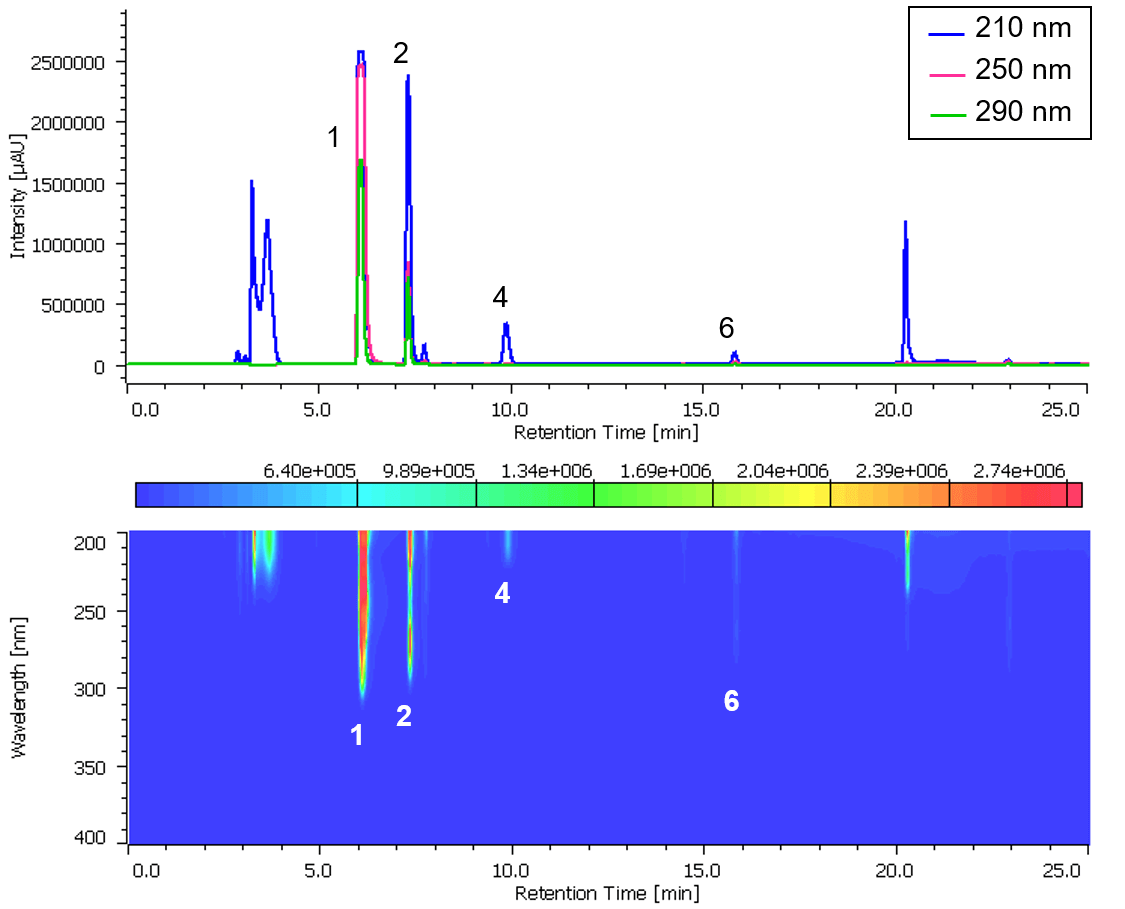
Figure 5. Chromatogram and contour plot of OTC drug for cold (A) (1: Acetaminophen, 2: Anhydrous caffeine, 3: Dihydrocodeine phosphate, 4: dl-Methylephedrine hydrochloride, 5: Ethenzamide, 6: Chlorpheniramine maleate, 7: Noscapine, 8: Glycyrrhizic acid, 9: Ibuprofen)
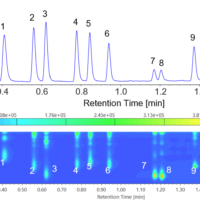
Chromatogram and contour plot of OTC drug for cold (B) are shown in Figure 6.
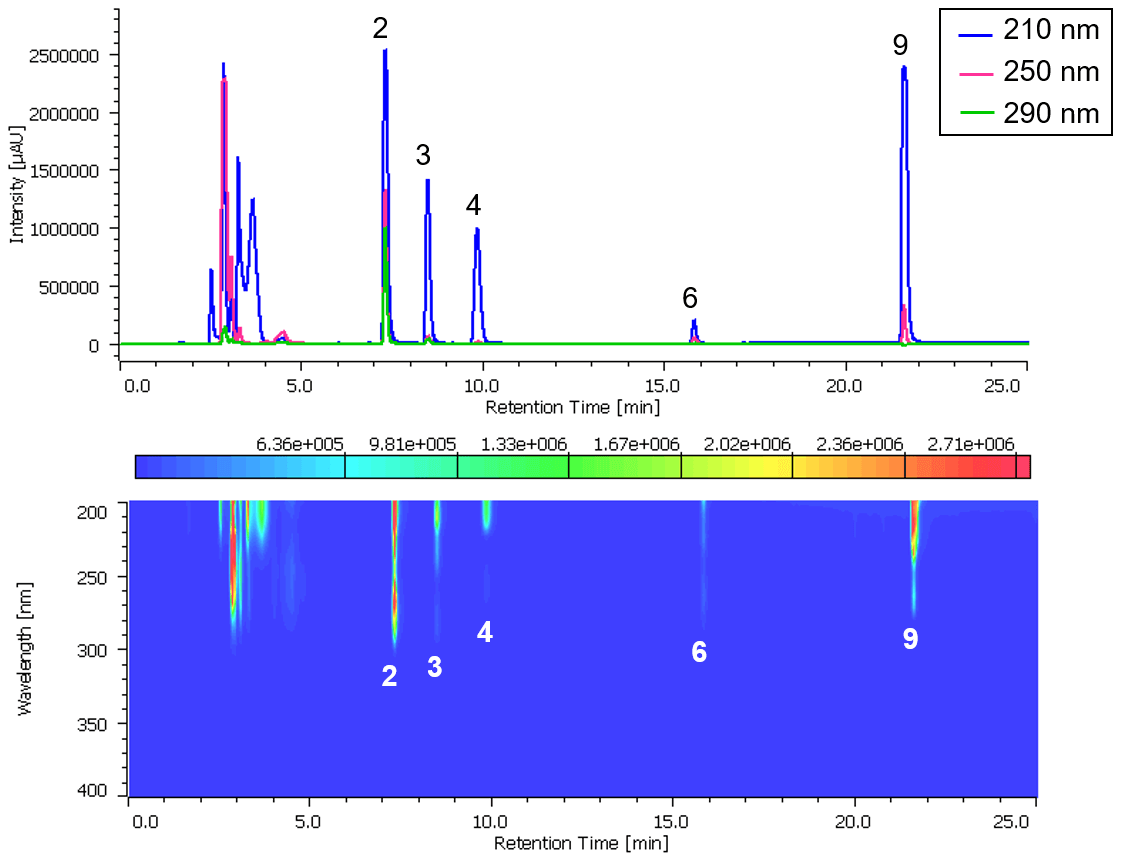
Figure 6. Chromatogram and contour plot of OTC drug for cold (B) (1: Acetaminophen, 2: Anhydrous caffeine, 3: Dihydrocodeine phosphate, 4: dl-Methylephedrine hydrochloride, 5: Ethenzamide, 6: Chlorpheniramine maleate, 7: Noscapine, 8: Glycyrrhizic acid, 9: Ibuprofen)