Introduction
Plastics that will not be decomposed in nature will eventually become microplastics and may affect ecosystems. Biodegradable plastics are attracting attention as a material that can reduce the burden on the global environment. Biodegradable plastics can be used in the same way as general plastics under normal use condition and are eventually decomposed into water and carbon dioxide by microorganisms when buried in the ground after use. Various evaluation methods have been reported for raw materials of biodegradable plastics so far*1 *2, but few cases have been evaluated for biodegradability of the final products. In this application data, using a commercially available garbage bags made of biodegradable plastic as a sample, biodegradation process was evaluated by FTIR.
Experimental
Sample
The soil burial test of a garbage bag made of biodegradable plastic (BS-PL copolymer: polybutylene succinate – polylactic acid copolymer) was carried out, and IR spectrum was measured consecutively. As a control, a general garbage bag (PE: polyethylene) was also tested and measured.
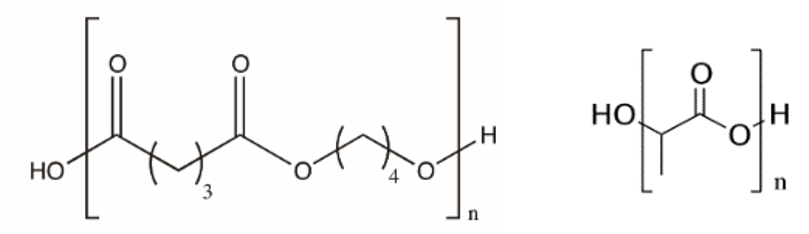
Structures of polybutylene succinate (left) and polylactic acid (right)
Soil burial test condition
Films cut into 3 cm x 6 cm was buried in a place about 5 cm deep in the soil at the burial place. (Films were measured in 2, 4, 8 weeks.)
Measurement condition
Instruments: FT/IR-4600
Resolution: 4 cm-1
Detector: DLATGS
Accumulation: 64 times
Measurement Method: ATR
Accessory: ATR PRO ONE (Diamond prism)

FTIR spectrometer with ATR Accessory (FT/IR-4600 + ATR PRO ONE)
Keywords
Biodegradable plastics, FTIR, ATR
Results
The day before the burial soil was taken as 0 day, the film was taken out after 0 day, 2 weeks, 4 weeks, 8 weeks, and the results of IR spectrum measurement is shown in Figure 1.
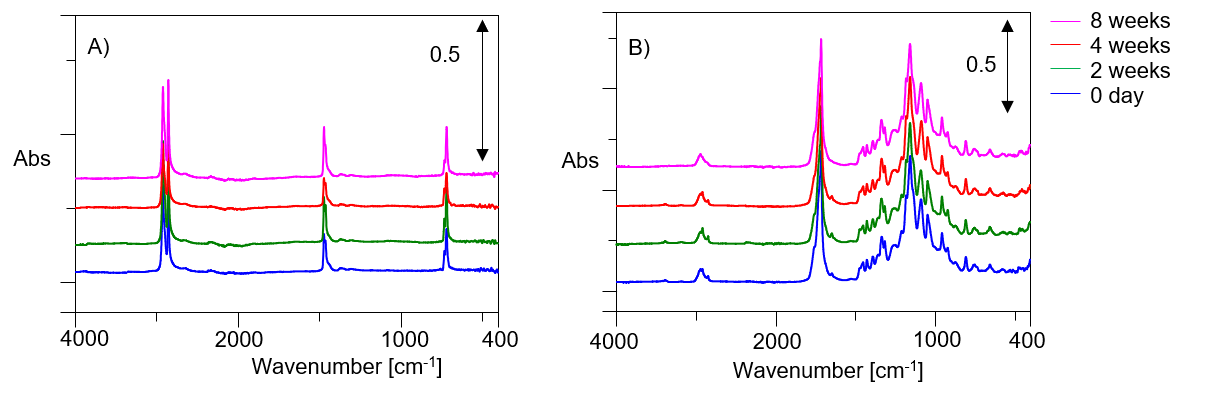
Figure 1. ATR-IR spectra of soil burial test (A: PE, B: biodegradable plastic) (offset)
In the soil burial test of a general garbage bag, no noticeable peak change was not recognized. On the other hand, in biodegradable plastics, the peak attributed to each functional group remarkably changed. Figure 2 shows an enlarged view of the area where the change was seen in Figure 1B. Changes were observed in the peak attributed to -CH2 group, -C═O and -C(═O)O-. Although data is not shown here, no noticeable change is observed in the transmission method.
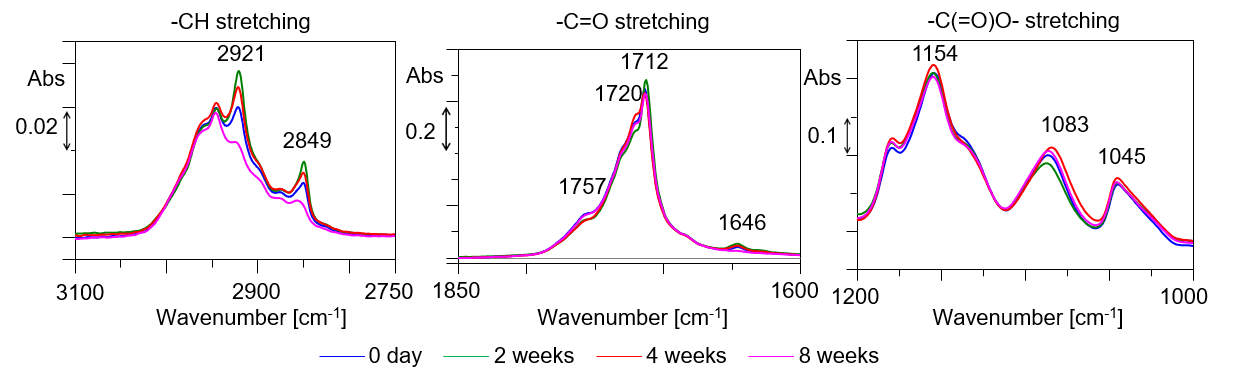
Figure 2 Sequential change of ATR-IR spectrum in each absorption band of biodegradable plastic (offset)
Since the change in the peak was observed only with the ATR method, it was confirmed that decomposition occurred from the surface with biodegradable plastics. In the stretching vibration of the -CH2 group near 3000 cm-1, it is considered that the biodegradable plastics are changed to dimers or monomers because the peak bandwidth decreases and sharp with the lapse of the time. Lowering of the peak of the -CH2 group thereafter may possibly capture the decomposition of biodegradable plastic from dimer and monomer in soil. On the other hand, the change in the peak attributed to -C=O, -C(═O)O- suggests a change in the carbonyl group due to cleavage of the ester bond.
Conclusion
The ATR method is considered to be effective for reaction analysis at the molecular level of products using biodegradable plastics since it was confirmed that decomposition of biodegradable plastics in the soil reacted from the surface. JASCO offers ATR accessories that can perform measurement while applying various perturbations such as heating and cooling. By combining these with field tests it is considered possible to analyze further reaction behaviors of biodegradable plastics.
References
1. Hiroyuki KUROKI, et al., The Field Test of Biodegradable Plastics, Report of Miyazaki Prefecture Industrial Technology Center & Miyazaki Prefectural Food & R&D Center, 46, 2001
2. Yun-Xuan Weng, et al., Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions, Polymer Testing, 32 (5), 2013.