Introduction
Ibuprofen is a well known non-steroidal anti-inflammatory drug and widely used for pain relief and fever reduction. Only the S enantiomer of ibuprofen has active antipyretic properties, but within the human body an enzymatic reaction can readily convert the R enantiomer into the S enantiomer.
The Circular Dichroism detector is well known for its high accuracy and selectivity for chiral analysis. Both CD and UV signal are measured simultaneously and spectral scanning can be used to optimize the wavelength of interest, which is important in the measurement of chiral compounds. Not only can the CD detector help differentiate between two enantiomers, but it can also distinguish between achiral impurities and enantiomers of interest.
LC-4000 HPLC system
Experimental
Chromatographic conditions
Column: CHIRALPAK AD-RH (4.6 mmI.D. x 150 mmL, 5 µm)
Eluent A: 0.1% phosphoric acid aqueous solution
Eluent B: Acetonitrile
Composition: Eluent A/B (60/40)
Flow rate: 0.5 mL/min
Column temp.: 25ºC
Wavelength: 230 nm
Response: 1.5 sec
Scan speed: 10 nm/sec
Injection volume: 10 µL
Standard sample: Mixture of 50 µg/mL Caffeine and 200 µg/mL racemic ibuprofen in eluent A/B (60/40)
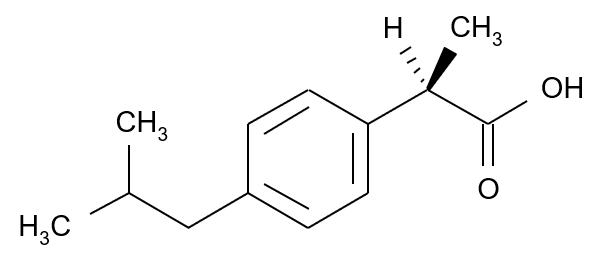
Ibuprofen
Results
Figure 1 shows chromatograms of caffeine and racemic ibuprofen standards. The UV chromatogram shows that both compounds are detected, but in the CD chromatogram only ibuprofen can be detected, as the caffeine is achiral. Figure 2 shows CD and UV spectra (measured by spectral scanning).
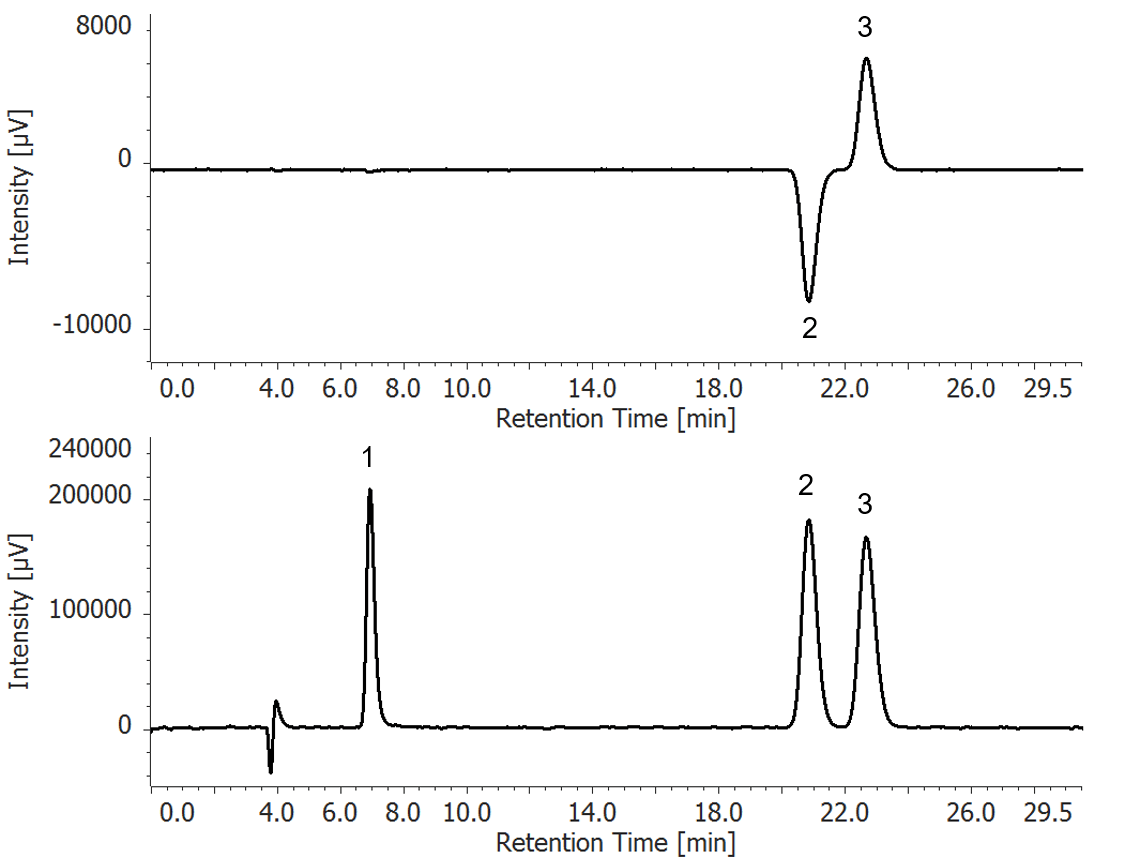
Figure 1. Chromatogram of Caffeine and Racemic Ibuprofen standards
(Top: CD detection, Bottom: UV detection, 1: Caffeine, 2: R-(-)-Ibuprofen, 3: S-(+)-Ibuprofen)
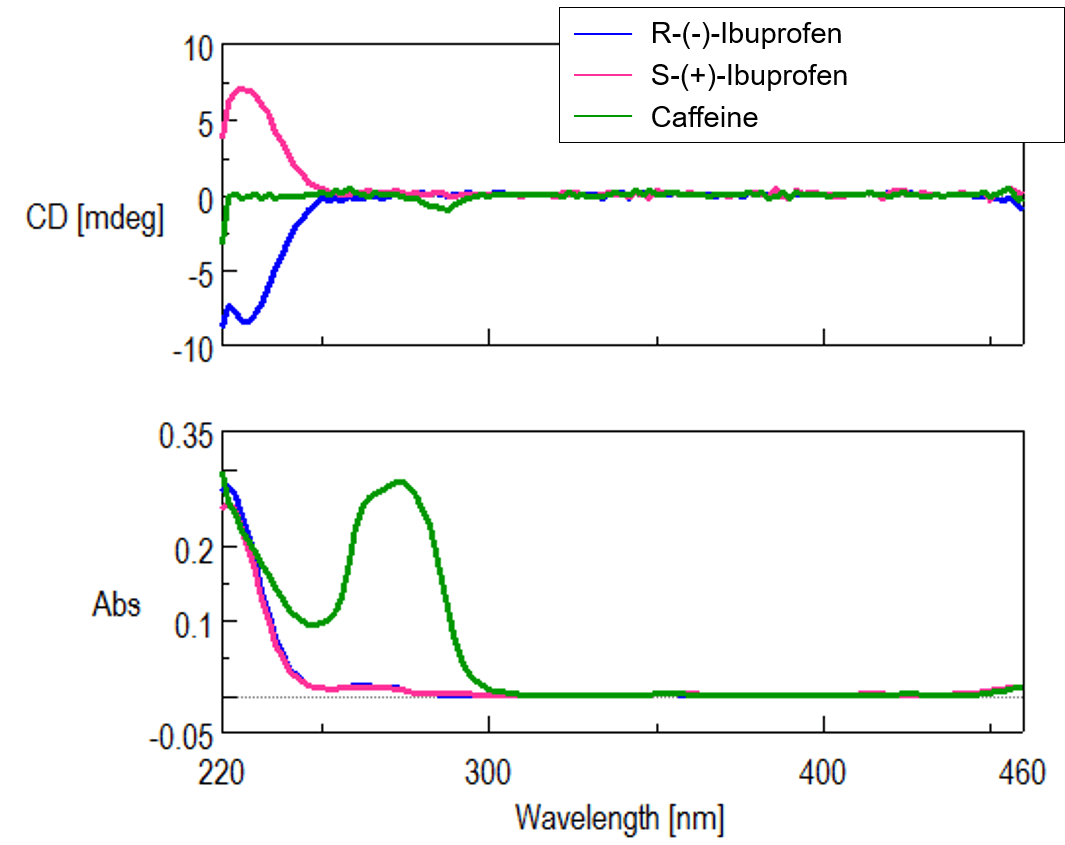
Fig. 2 Spectral Measurement (Top: CD, Bottom: UV)