Introduction
Since fat-soluble vitamins are easily dissolved in oils and fats, there is little loss of fat-soluble vitamins due to washing with water or heating, and cooking with oils and fats greatly increases the absorption rate at ingestion. However, excessive fat-soluble vitamins will be stored and accumulated in the liver, adipose tissue, etc., so it is necessary to be careful about hypervitaminosis. Vitamins A, D, E and K corresponding to fat-soluble vitamins are pointed out as possibility of disease or growth disorder due to overdose or deficiency. For example, with regard to vitamins A, D, and K, excessive intake may cause symptoms such as headache (vitamin A), nauseous (vitamin A), rough skin (vitamin A), kidney stone (vitamin D) and anemia (vitamin K).
Meanwhile, vitamin E acts as an antioxidant for fat-soluble substances in vivo, because it is very easily oxidized after absorption. Due to this antioxidant action, vitamin E is said to be a rejuvenating vitamin.
This article shows the separation of 8 fat-soluble vitamins by using supercritical fluid chromatography (SFC) system.

LC-4000 SFC system
Experimental
Experimental conditions
Column: SFCpak Crest C18T-5 (4.6 mmI.D. x 250 mmL , 5 μm)
Eluent : CO2 / Modifier
Modifier solvent: Acetonitrile/Methanol(50/50)
Gradient: (CO2/Mod), 0 min(95/5)→2 min(95/5)→10 min(70/30)→12 min(70/30)→12.1 min(95/5) 1cycle; 17 min
Flow rate: 3.0 mL/min
Column temp.: 30 ˚C
Wavelength: 200 ~ 400 nm (220 nm: Vitamin E/E acetate, 248 nm: Vitamin K1/K3, 264 nm: Vitamin D2/D3, 320 nm: Vitamin A acetate/A palmitate)
Pressure: 15 MPa
Injection volume: 5 μL
Standard sample: 8 fat-soluble vitamins 100 mg/L each in ethanol
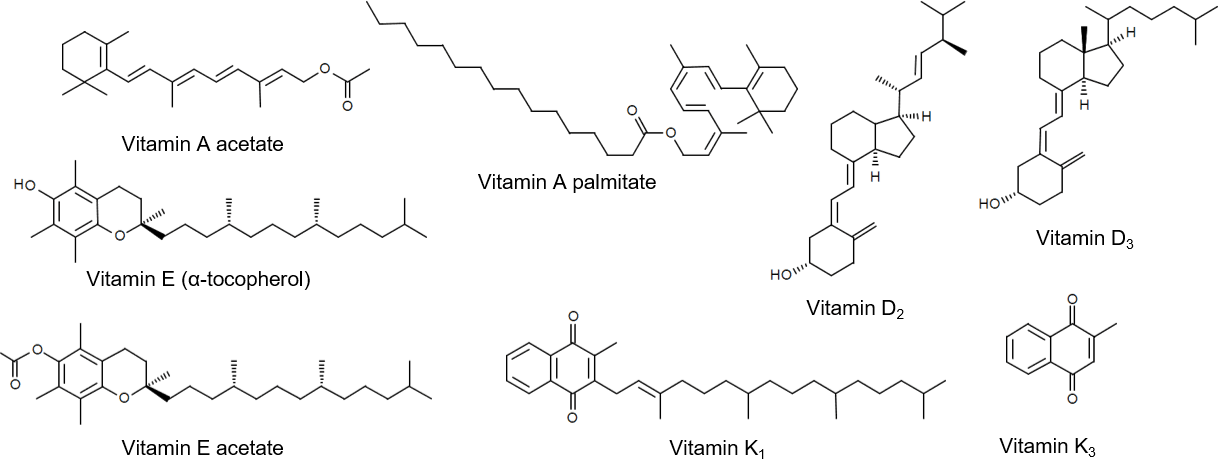
Structure
Keywords
SFC, fat-soluble vitamin, SFCpak Crest C18T-5, PDA detector
Results
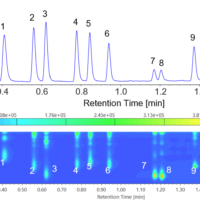
Figure 1 shows the contour map of the standard solution. A good separation of 8 components within 8 minutes was achieved.
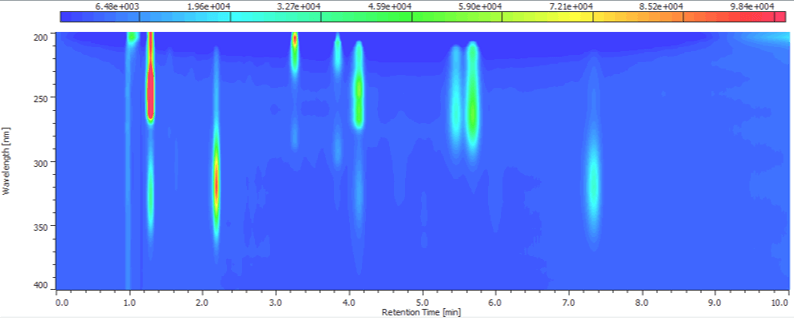
Figure 1 Contour map of standard solution ( 1: Vitamin K3, 2: Vitamin A acetate, 3: Vitamin E acetate, 4: Vitamin E, 5: Vitamin K1, 6: Vitamin D2, 7: Vitamin D3, 8: Vitamin A palmitate)
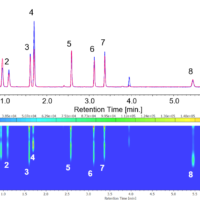
Figure 2 show the chromatograms of each wavelength.
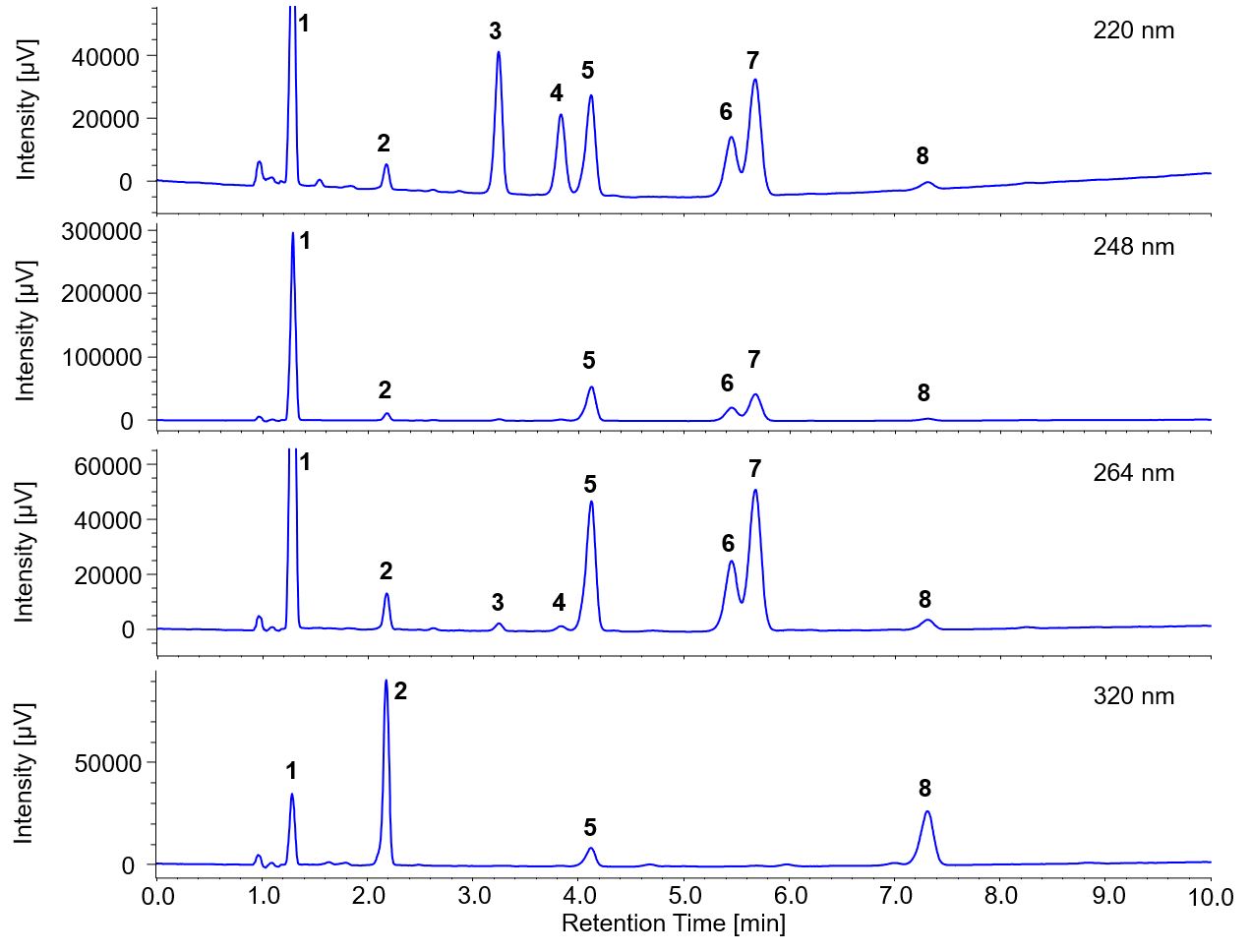
Figure 2. Chromatograms of standard solution (1: Vitamin K3, 2: Vitamin A acetate, 3: Vitamin E acetate, 4: Vitamin E, 5: Vitamin K1, 6: Vitamin D2, 7: Vitamin D3, 8: Vitamin A palmitate)
On-peak spectrum of each components is shown in Figure 3.
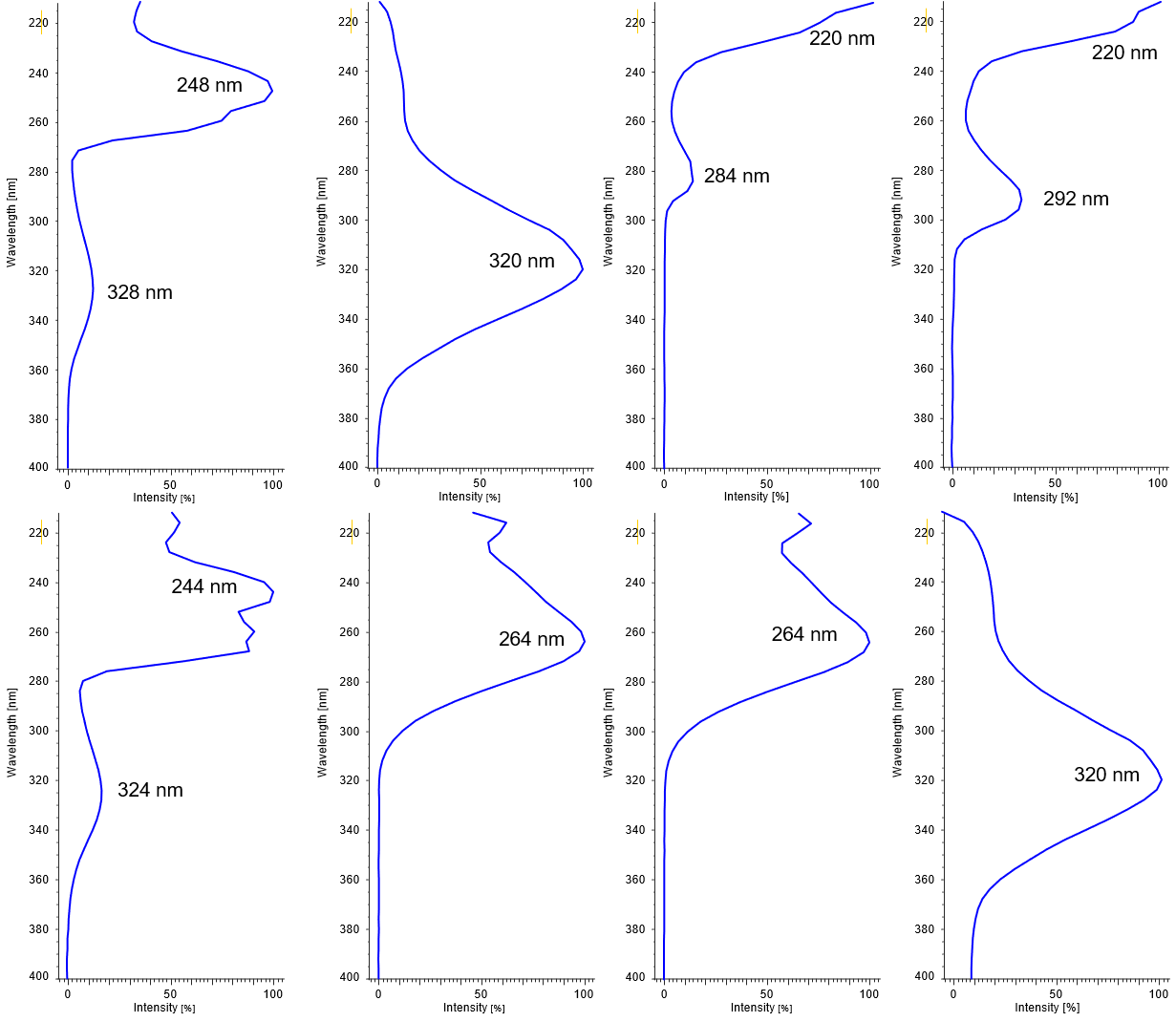
Figure 3. On-peak spectra of each component (1: Vitamin K3, 2: Vitamin A acetate, 3: Vitamin E acetate, 4: Vitamin E, 5: Vitamin K1, 6: Vitamin D2, 7: Vitamin D3, 8: Vitamin A palmitate)