Introduction
Vitamins are nutrients essential for assisting the metabolism within the body and maintaining normal bodily functions. Since they cannot be synthesized within the body to any significant extent, they must be obtained from food sources. In recent years, many health foods containing vitamins have also become commercially available. Water-soluble vitamins such as the vitamin B group and vitamin C dissolve in bodily fluids like blood and assist the function of enzymes necessary for various metabolic processes within the body.
In this study, we performed simultaneous analysis of ten water-soluble vitamins by high performance liquid chromatography (HPLC) using a photodiode array (PDA) detector.
LC-4000 series HPLC system
Experimental
Instruments
Pump: PU-4180*
Autosampler: AS-4050*
Column oven: CO-4060
Detector: MD-4015
* with option units
Conditions
Column: Unifinepak C18 (4.6 mmI.D. x 150 mmL, 5 µm)
Eluent A: 10 mmol/L sodium dihydrogen phosphate aqueous solution (adjusted to pH 2.8 with phosphoric acid)
Eluent B: Acetonitrile
Gradient: A/B = 99/1 (0.0 min) -> 75/25 (10.0 min) -> 75/25 (15.0 min) -> 99/1 (15.1 min), 1cycle; 20 min
Flow rate: 1.0 mL/min
Column temp.: 40 ºC
Wavelength: 210, 260 nm
Injection volume: 20 µL
Standard sample
Standard reagents:
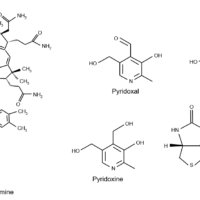
Pyridoxamine hydrochloride, thiamine hydrochloride, L-ascorbic acid, nicotinic acid, pyridoxal hydrochloride, nicotinamide, pyridoxine hydrochloride, calcium pantothenate, folic acid, riboflavin
Standard stock solution:
1) Pyridoxamine, thiamine, nicotinic acid, pyridoxal, nicotinamide, pyridoxine, pantothenic acid equivalent to 10 mg each dissolved in ultrapure water, and then made up to 10 mL (1000 mg/L).
2) 10 mg of L-ascorbic acid dissolved in 5% metaphosphoric acid solution and make up to 10 mL (1000 mg/L).
3) 10 mg each of folic acid and riboflavin dissolved in 0.5 mL of 100 mmol/L sodium hydroxide solution, and then made up to 10 mL with ultrapure water (1000 mg/L).
Calibration solutions:
Standard stock solutions diluted with 10 mmol/L phosphate buffer (pH 6.9) to obtain concentrations of 1.0, 2.5, 5.0, 10, and 25 mg/L.
Unknown sample
Multivitamin tablet (pre-treatment method shown in Figure 3)
Structure
Keywords
Water-soluble vitamins, vitamin B group, vitamin C, HPLC, Unifinepak, C18 column, PDA detector
Results
Figures 1 show the chromatograms of the calibration solution for 10 mg/L water-soluble vitamins obtained by HPLC with a PDA detector at detection wavelengths of 210 and 260 nm, respectively. Figure 2 shows the corresponding HPLC contour plot. Table 1 shows the reproducibilities of the retention times and peak areas, the correlation coefficients for the calibration curves (each from 1.0 to 25 mg/L), the detection limit, and the quantification limit. For all components, good results were obtained, with reproducibilities of ≤ 0.1% for retention time, and ≤ 2% for peak area. The calibration curve exhibited good linearity, with a correlation coefficient (r) of ≥ 0.9990.
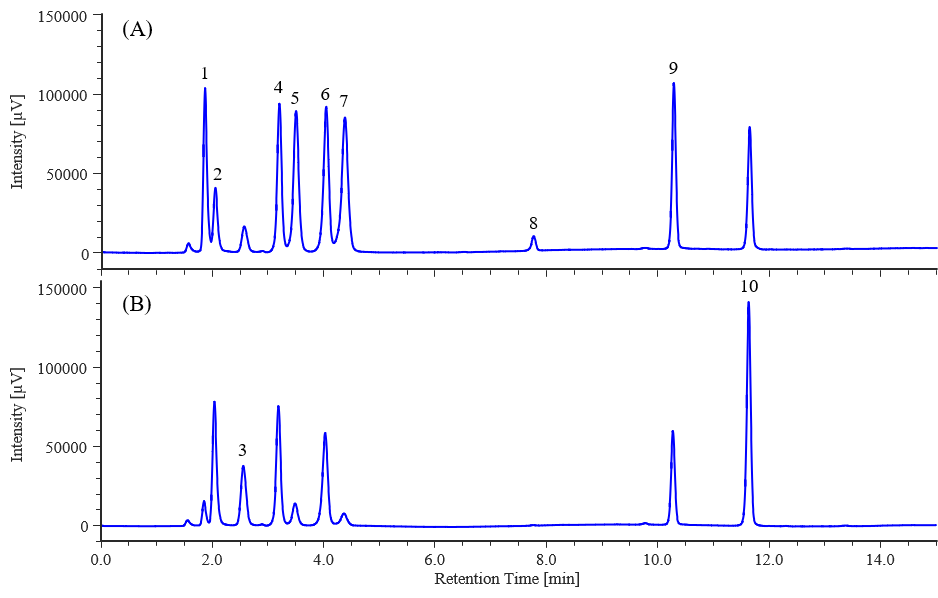
Fig. 1 Chromatograms of water-soluble vitamins (10 mg/L each) (A): 210 nm, (B): 260 nm
1: Pyridoxamine, 2: Thiamine, 3: L-ascorbic acid, 4: Nicotinic acid, 5: Pyridoxal, 6: Nicotinamide, 7: Pyridoxal, 8: Pantothenic acid, 9: Folic acid, 10: Riboflavin
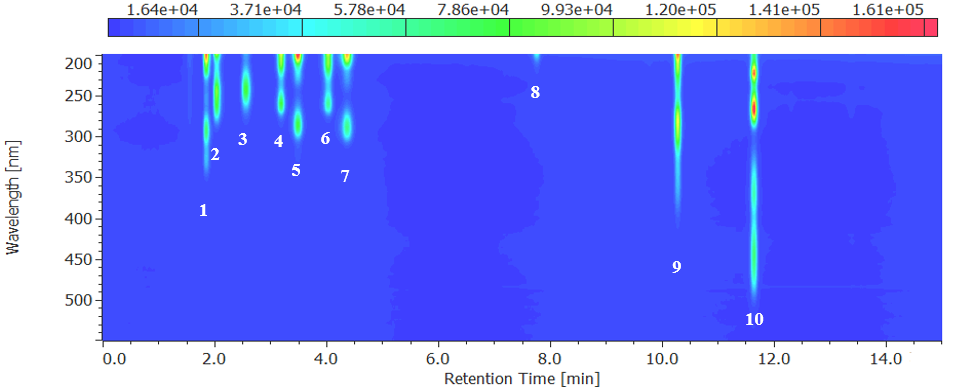
Fig. 2 Contour plot for water-soluble vitamins (10 mg/L each)
1: Pyridoxamine, 2: Thiamine, 3: L-ascorbic acid, 4: Nicotinic acid, 5: Pyridoxal, 6: Nicotinamide, 7: Pyridoxal, 8: Pantothenic acid, 9: Folic acid, 10: Riboflavin
Table 1 Reproducibility, correlation coefficient, detection limit, and quantification limit for calibration solutions
| Analysis species | Relative Standard Deviation [%] *1 | Correlation coefficient (r) | Detection limit [ng] *2 (S/N = 3) |
Quantification limit [ng] *2 (S/N = 10) |
|
| Retention time | Peak area | ||||
| Pyridoxamine | 0.00 | 0.94 | 0.9990 | 0.75 | 2.51 |
| Thiamine | 0.08 | 0.99 | 0.9998 | 1.20 | 3.99 |
| L-ascorbic acid | 0.07 | 1.16 | 0.9998 | 1.61 | 5.38 |
| Nicotinic acid | 0.06 | 0.82 | 1.0000 | 0.58 | 1.93 |
| Pyridoxal | 0.08 | 1.02 | 0.9999 | 0.62 | 2.07 |
| Nicotinamide | 0.08 | 0.92 | 1.0000 | 0.61 | 2.02 |
| Pyridoxine | 0.09 | 1.20 | 0.9998 | 0.69 | 2.30 |
| Pantothenic acid | 0.08 | 0.75 | 1.0000 | 4.69 | 15.6 |
| Folic acid | 0.05 | 0.69 | 0.9999 | 0.52 | 1.72 |
| Riboflavin | 0.05 | 0.61 | 0.9999 | 0.39 | 1.30 |
*1 Calculated from the measurement results for each 10 mg/L calibration solution
*2 Calculated from the measurement results for each 1 mg/L calibration solution
Figure 3 shows the pretreatment procedure for a multivitamin tablet.
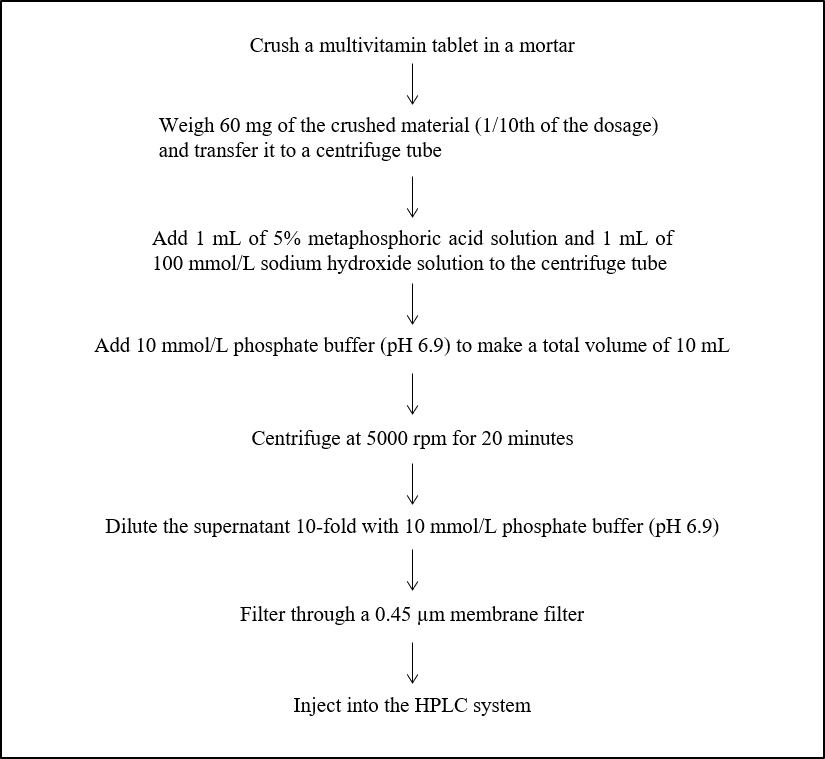
Fig. 3 Pretreatment procedure for a multivitamin tablet
Figure 4 and 5 show chromatograms and contour plots of the multivitamin tablets, respectively, and Table 2 shows the quantitative values. The quantitative values confirmed that the results were generally equivalent to the values actually displayed.
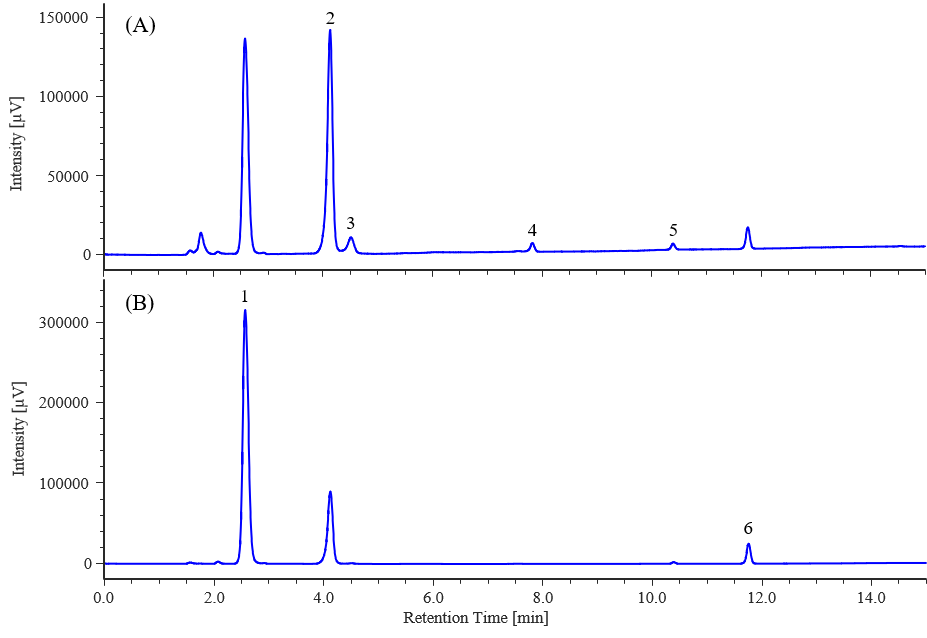
Fig. 4 Chromatograms of multivitamin tablet (A): 210 nm, (B): 260 nm
1: L-ascorbic acid, 2: Nicotinic acid, 3: Pyridoxine, 4: Pantothenic acid, 5: Folic acid, 6: Riboflavin
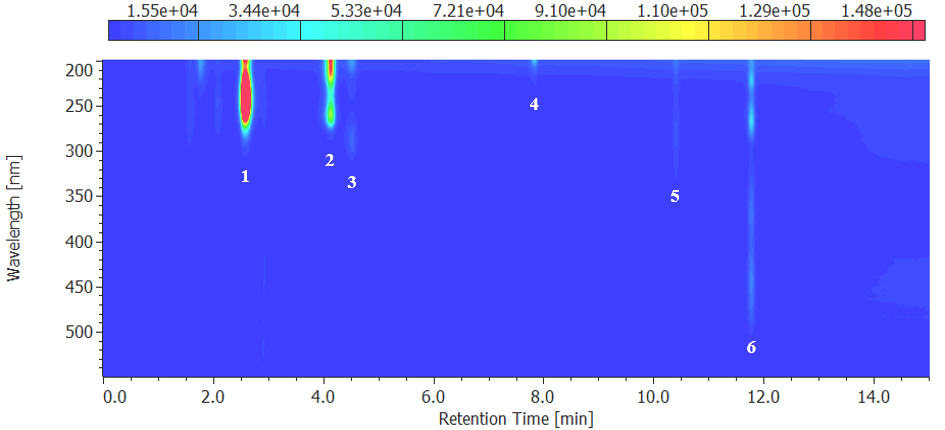
Fig. 5 Contour plot for multivitamin tablet
1: L-ascorbic acid, 2: Nicotinic acid, 3: Pyridoxine, 4: Pantothenic acid, 5: Folic acid, 6: Riboflavin
Table 2 Quantitative values for multivitamin tablet
| Analysis species | Quantitative value [mg]* | Display value [mg]* |
| L-ascorbic acid | 87 | 100 |
| Nicotinamide | 15 | 13 |
| Pyridoxine | 1.3 | 1.3 |
| Pantothenic acid | 4.5 | 4.8 |
| Folic acid | 0.27 | 0.24 |
| Riboflavin | 1.5 | 1.4 |
* Amount per 600 mg
Conclusion
In this study, simultaneous analysis of ten water-soluble vitamins was performed using a C18 column and PDA detection. Measurements using calibration solutions confirmed good separation of all components, good reproducibility of retention times and peak areas, and good linearity of the calibration curves. Furthermore, when multivitamin tablets were treated as unknown samples and processed and measured, six water-soluble vitamin components were detected. Calculating the amounts of these components based on the created calibration curve yielded results that generally matched the values actually displayed on the product. This measurement method is applicable for the quantitative evaluation of water-soluble vitamins in food.