Introduction
Near-Infrared (NIR) spectroscopy has unique capabilities as compared to mid-infrared analysis which can often require extensive sample preparation to obtain an identifiable spectrum. As such, NIR can be useful for the non-destructive analysis of a specific sample area or provide an average of a larger sample area, depending upon the required analysis. In recent years, NIR spectroscopy has been widely used for the examination of biological samples and quality control/analysis of food and medical products. The diffuse reflectance technique is ideal for this method due to extremely simple sample handling such that rapid identification of illegal drugs, such as MDMA, can be accomplished by using a search data library created using NIR diffuse reflectance.
Experimental
A diffuse reflection accessory is used in combination with a portable Fourier Transform Near-Infrared Spectrometer (VIR-300) and then by simply placing a tablet, such as MDMA, directly on the sample holder, a measurement can be performed without further sample preparation. An InGaAs detector is used to provide enhanced sensitivity and a rapid scanning capability. Principal Components Analysis (PCA) was performed to simplify positive identification of an MDMA tablet. When the possible grouping of spectral data was confirmed based on the PCA program, a library was established. For establishing the PCA data library, 40 types of tablets were analyzed, namely 25 types of over-the-counter pharmaceuticals, such as gastrointestinal drugs, one type of amphetamine (AP), eight types of MDMA (street name: ecstasy), three types of methamphetamine (MA), and three types of MDA (street name: the love drug).

Figure 1. VIR-300 and the diffuse reflection accessory
The utility of a simple identification system was examined by the investigation of the algorithm, the calculation parameters and a threshold established from comparison of the search results from randomly selected tablets. The tablets were placed on the sample holder directly, as shown in Figure 2 (left). In the case of an extremely small tablet, unable to be placed on the holder, the sample was placed in a test tube like the one shown in Figure 2 (right), and measured.
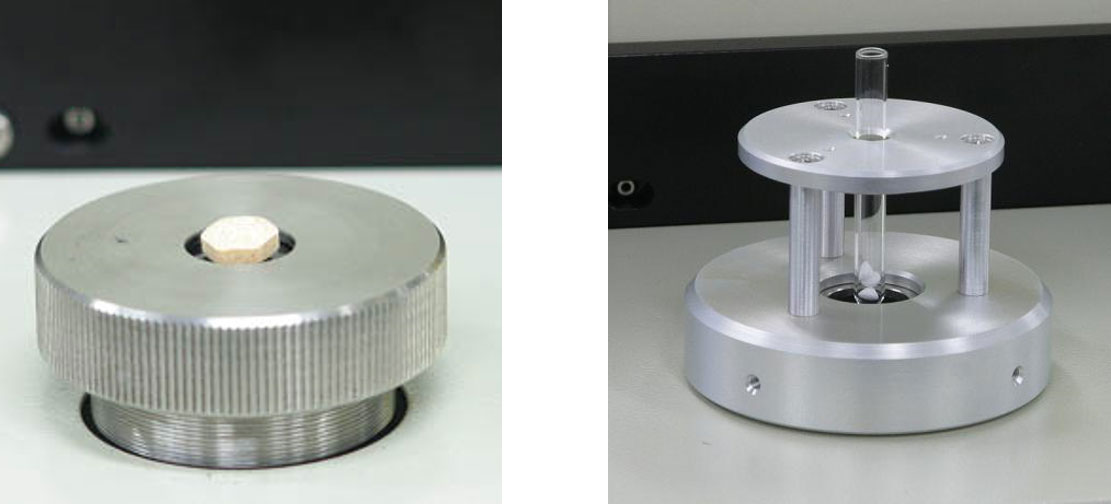
Figure 2. Measurements of a tablet (left) and crushed/powdered samples (right)
Results
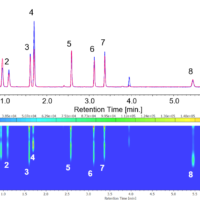
Figure 3 (right) illustrates the PCA analysis results for an MDMA tablet, and Figure 3 (left) includes examples of the Near-Infrared spectra for the 4 classes of illegal drugs.
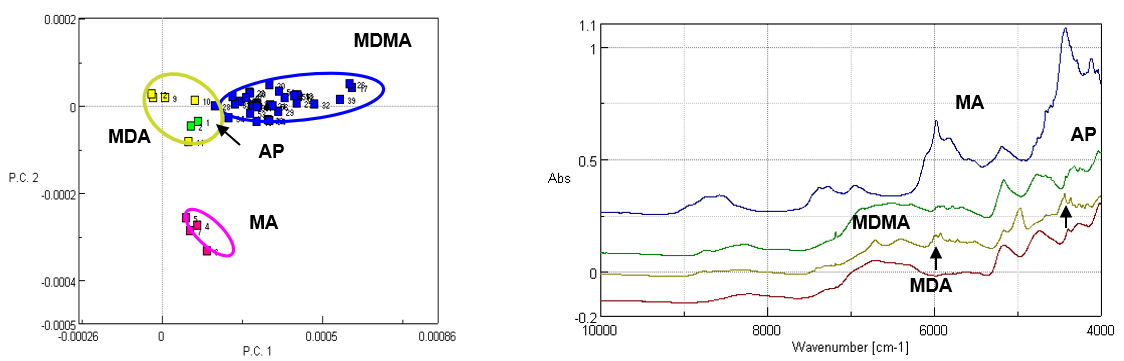
Figure 3. PCA analysis results for street drugs (left) and diffuse reflectance spectra of various street drugs (right)
Sample identification can be accomplished by using the region (indicated by the arrow) where the spectral absorptions can provide specific peaks depending on the tablet component. The PCA method, however, provides distinct discrimination between the various drug components due to subtle variations in the NIR spectra. Since NIR spectra do not provide specific discriminatory peaks like mid-IR spectra, the PCA method offers an enhanced discrimination of the various drug components, strengthening the identification of the “unknown” drug tablet. The NIR diffuse reflection system is ideal as a rapid analysis method because the tablet is simply placed on the accessory platform and the required sample measurement time is only 10 seconds. This method will become more powerful for sample identification as the library data is expanded with additional standard sample data.
Figure 4 demonstrates a calibration model illustrating the correlation between tablets containing MDMA and quantitative results of these tablets using GC analysis.
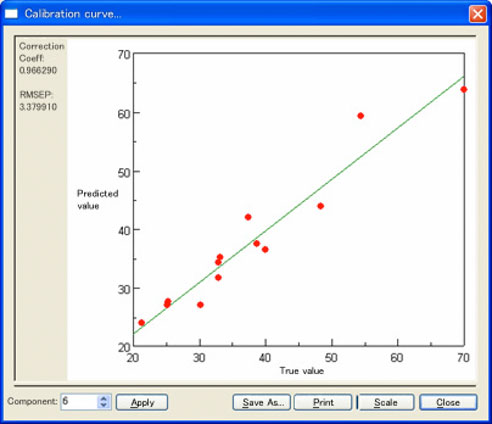
Figure 4. Calibration curve of NIR and GC results for MDMA
With a correlation coefficient of R=0.966, these results demonstrate a sufficient correlation for performing sample quantitation. This would make it possible to analyze the amount of MDMA in illicit tablets by linking the search results of the identification program with the calibration model developed using GC analysis. Figure 5 is an example of the program developed for the identification of the drug formulations using the PCA search method.
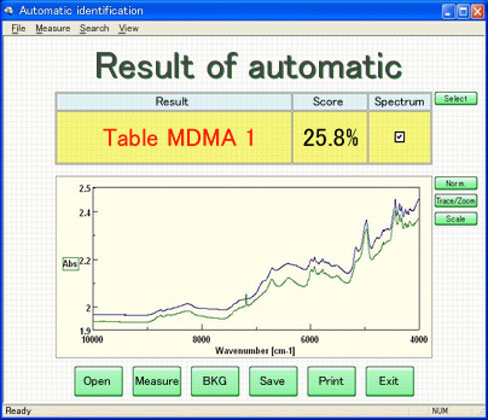
Figure 5. Confirmation test and quantitative analysis results
Conclusion
We have demonstrated the use of a NIR analysis methodutilizing PCA discrimination for the identification of various drug formulations. The NIR system demonstrates a rapid, nondestructive analysis method for various drug tablets that can be extended to provide quantitative analysis of the drug concentration.