Introduction
FRET is the mechanism that the energy is transferred non-radiatively from an excited state donor to a neighbor acceptor (Figure 1). FRET is an abbreviation for Forester (or fluorescence) resonance energy transfer. Since FRET typically occurs when the distance between the donor and acceptor is 1–10 nm, it enables to monitor the structure change of protein and the interaction between specific proteins by binding donor and acceptor to the target protein.
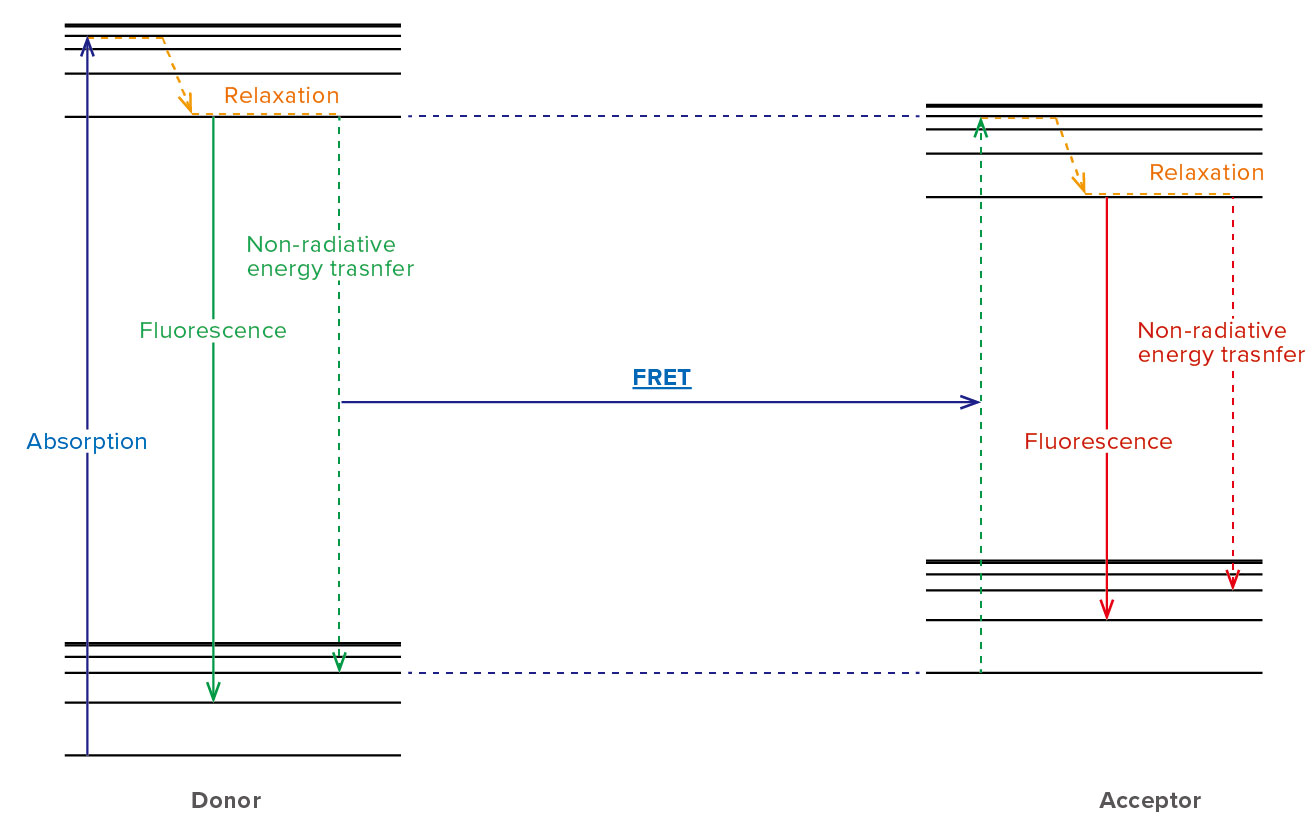
Figure 1. Energy transition in a FRET pair
FRET is monitored by the spectrofluorometer, which measures the fluorescence/quenching of acceptor or excited donor. FRET efficiency depends on the following factors (Figure 2).
1. Spectral overlap between the donor and acceptor As the overlap area of the donor’s fluorescence spectrum and the acceptor’s absorption spectrum is larger, FRET efficiency is higher.
2. Distance between donor and acceptor
FRET efficiency is inversely proportional to the six power of distance between the donor and acceptor.
3. Orientation of donor and acceptor
FRET efficiency is a maximum when the two dipole moments are parallel or anti-parallel to each other, and no energy transfer occurs when the dipole moments are perpendicular to each other.
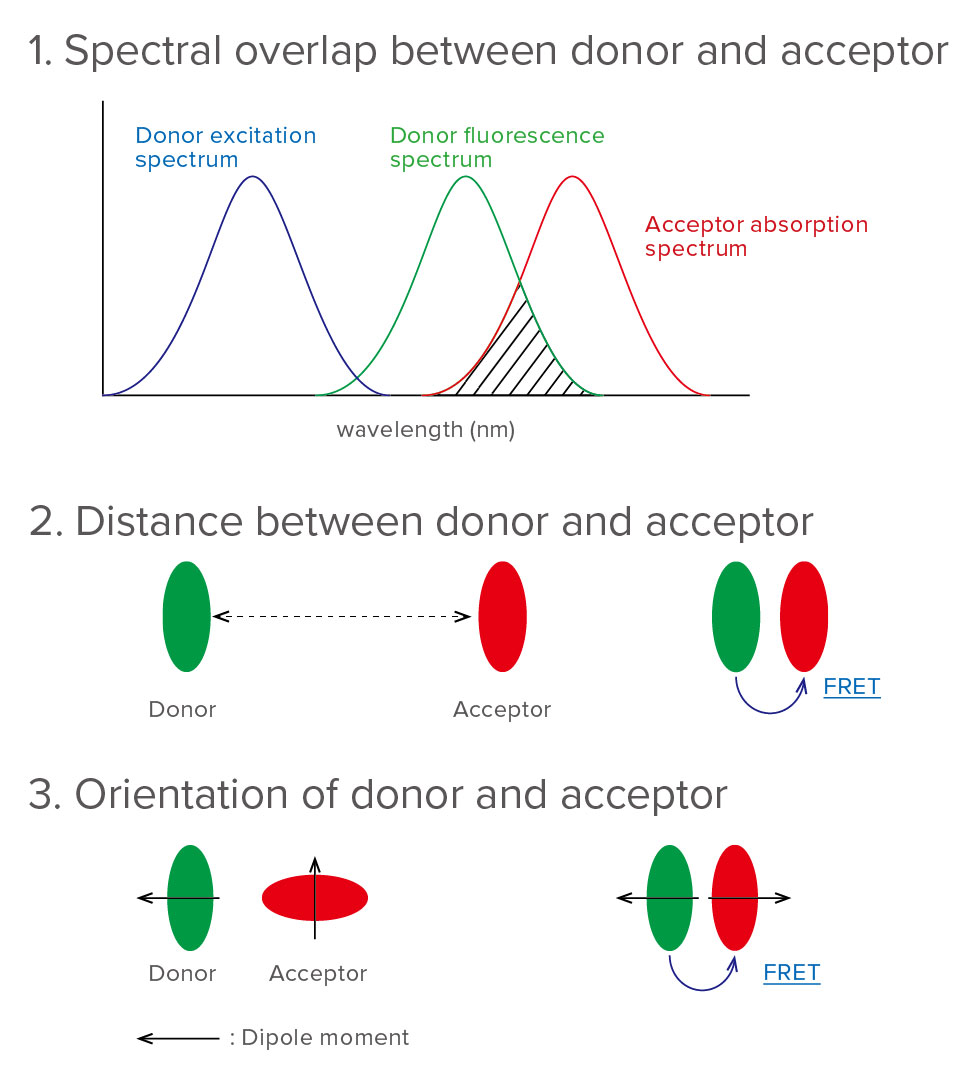
Figure 2. Factors that influence FRET
One of monitoring ways of protein behavior by using FRET is a cyan fluorescent protein (CFP) – yellow fluorescent protein (YFP) pair.
CFP and YFP, which are color variants of green fluorescent protein (GFP), are well-known as the fluorescent protein that FRET occurs. CFP absorbs excitation light of 433 nm, and produces fluorescence light of 476 nm. When CFP is close to YFP, the energy which CFP has absorbed is transferred to YFP by the interaction between CFP and YFP, and YFP produces fluorescence light of 527 nm (Figure 3) [1].
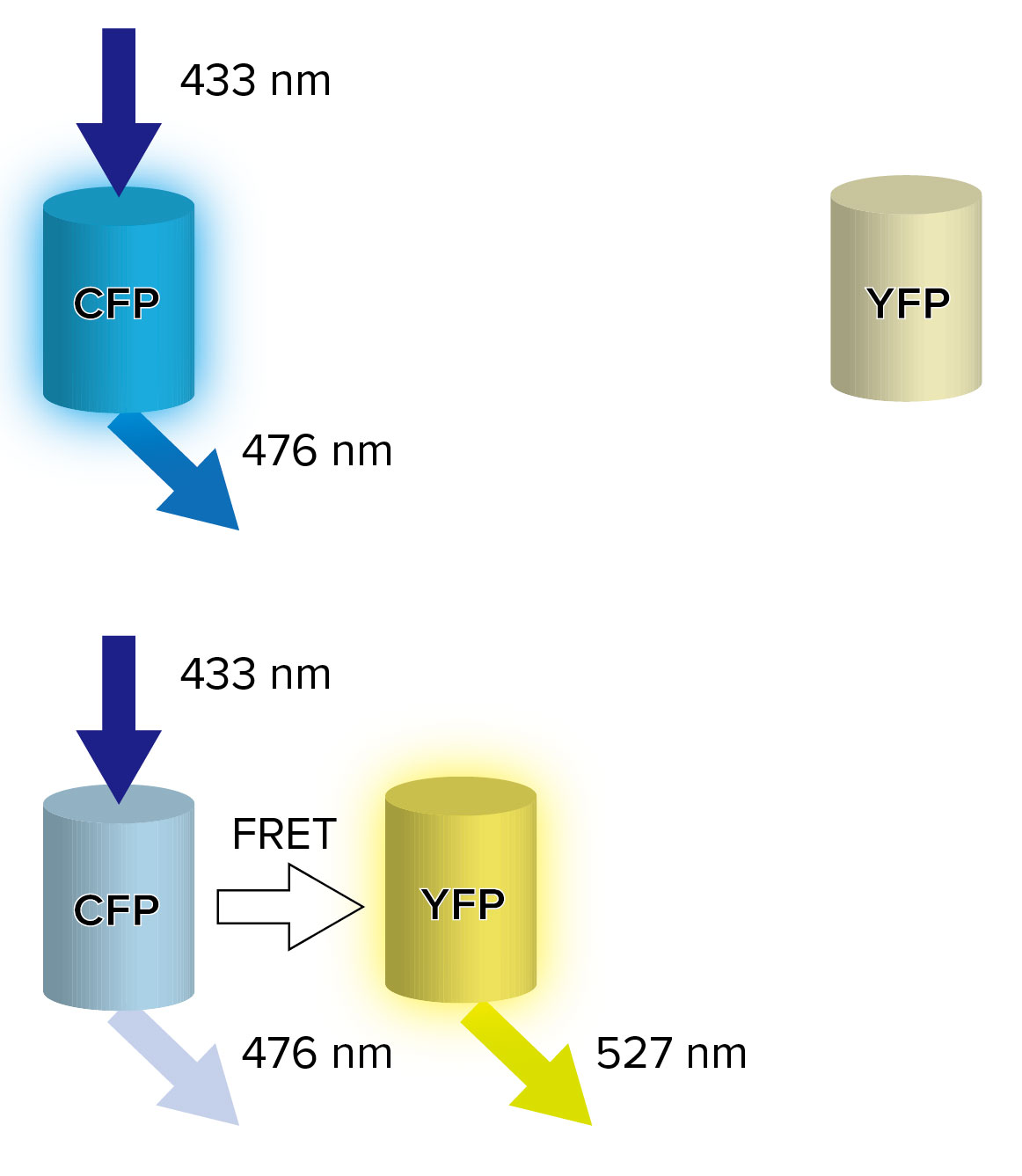
Figure 3. FRET between CFP and YFP
Its property can be applied to protein behavior monitoring by binding CFP and YFP to the target protein.
Fusing CFP and YFP to two different host proteins enables to monitor the protein-protein interactions (Figure 4. intermolecular FRET), and fusing CFP and YFP to two different sites in the same protein enables to monitor the protein conformational change (Figure 4. intramolecular FRET).
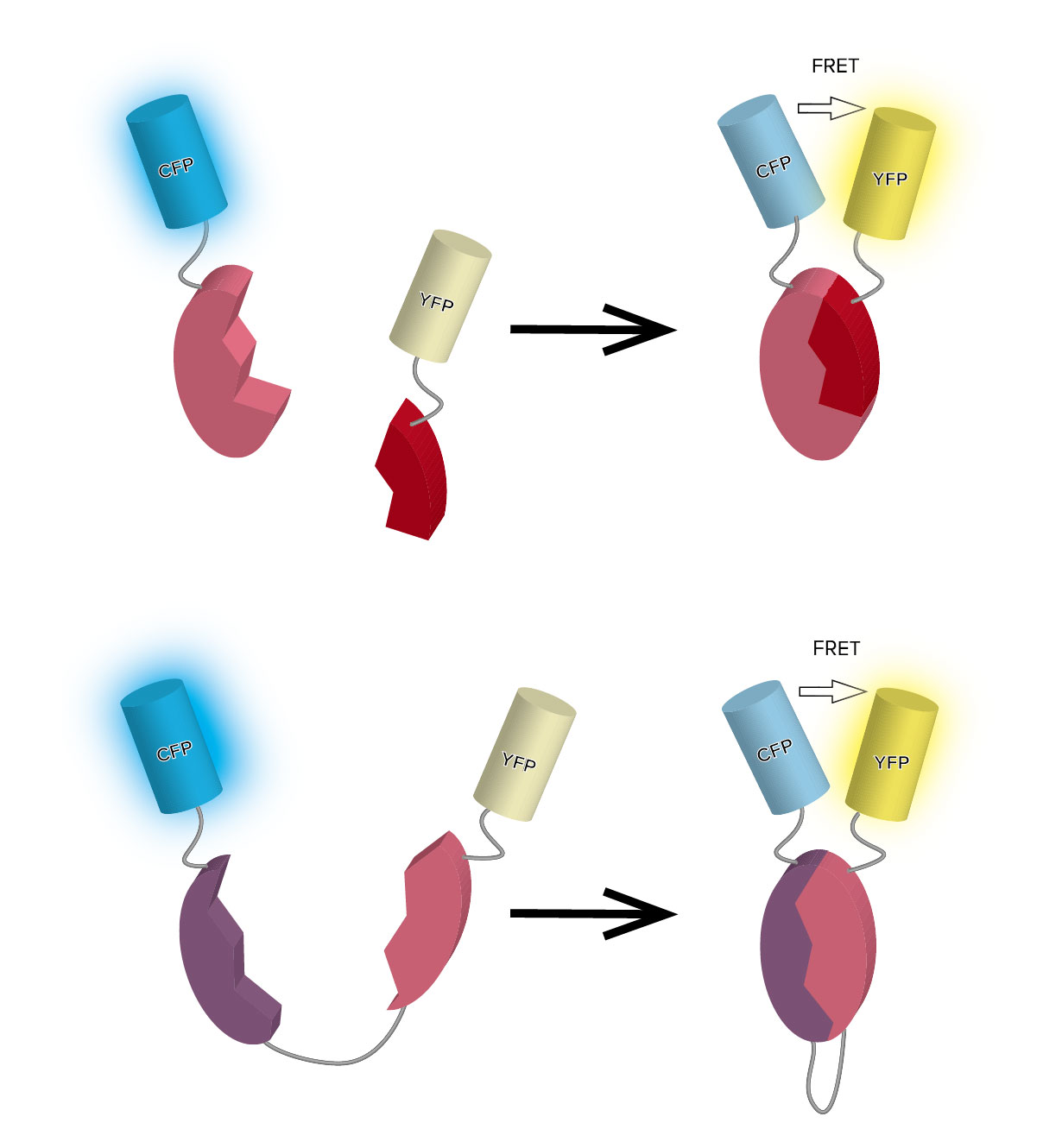
Figure 4. Intermolecular FRET (top) and Intramolecular FRET (bottom)
This property can be also applied to biosensor. One of its applications is the biosensor by using DNA aptamer, which has the capability to bind a specific
molecule.
DNA aptamer, whose ends are fused donor/acceptor fluorescent molecules, can detect the target molecule because FRET occurs by changing its structure when binding DNA aptamer to target molecule (Figure 5).
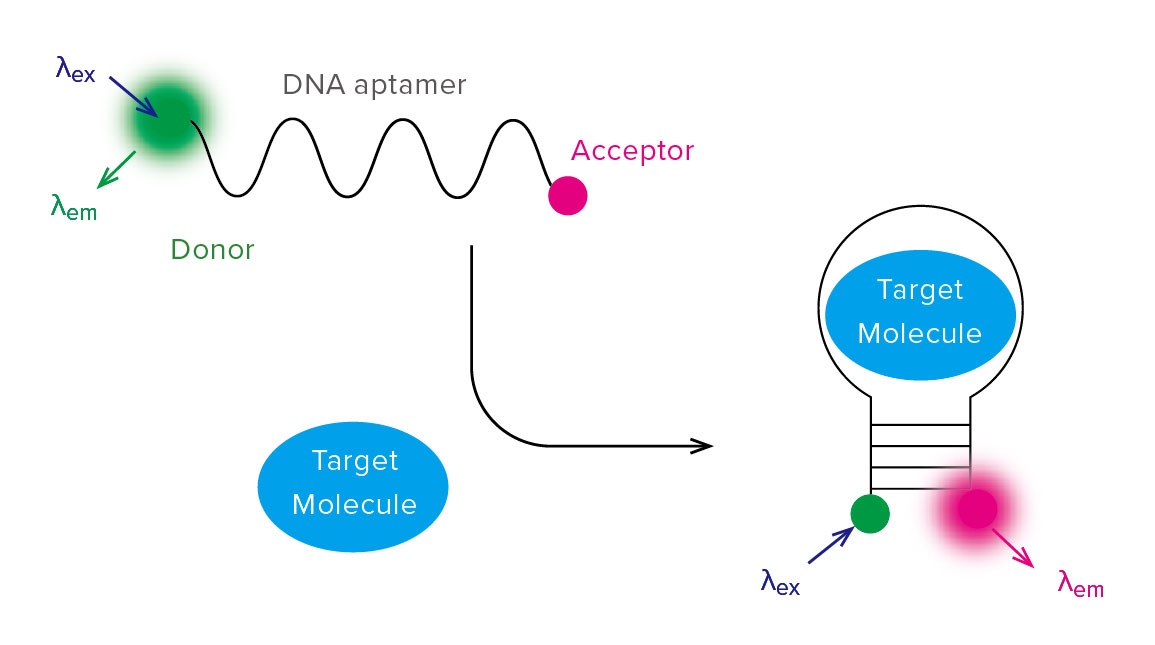
Figure 5. Schematic diagram of biosensor by using DNA aptamer
JASCO’s spectrofluorometer can detect FRET phenomenon with high sensitivity, and provide its useful information to the user.

FP-8350 Spectrofluorometer
References
[1] Roger Y. Tsien 2009 Constructing and Exploiting the Fluorescent Protein Paintbox (Nobel Lecture). Angew. Chem. Int. Ed. 48: 5612–5626