Introduction
Cortisone acetate, a steroid, is administered to reduce tissue inflammation or to suppress the human immune system. While several pharmacopoeias say that its quality should be verified by HPLC analysis, there are a lot of demands that it wants to be verified faster than the conventional method (HPLC).
This article shows the utility of an X-PressPak C18S column (2.1 mm I.D. x 50 mm L.) packed with 2 µm diameter packing material for the ultra-high speed separation of the above steroid drug.

LC-4000 UHPLC system
Experimental
Chromatographic conditions
Column: X-PressPak C18S (2.1 mm I.D. x 50 mmL, 2 µm)
Mobile phase: CH3CN/H2O (35/65)
Column temperature: 25 ºC
Flow rate: 0.7 mL/min
Detection wavelength: 254 nm
Injection volume: 1 µL
Results
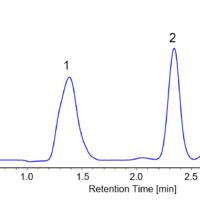
Figure 1 shows the separation of a standard mixture of propyl paraben (0.03 mg/mL), butyl paraben (0.03 mg/mL) and cortisone acetate (0.1 mg/mL). The UHPLC system provides an analysis time 4 times shorter than conventional HPLC while the resolution between the propyl paraben and cortisone acetate elutions was 12.2; the reproducibility of the peak ratio is 0.44%.
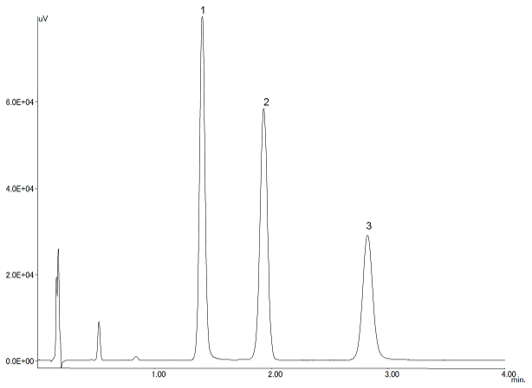
Figure 1. UHPLC chromatogram of a standard mixture (1=propyl paraben (0.03 mg/mL), 2=butyl paraben (0.03 mg/mL), 3=cortisone acetate (0.1 mg/mL))