Introduction
Infrared spectroscopy is an effective method for analysing the composition of biological samples such as proteins, sugars, and lipids, and also interactions between them. However, using conventional Fourier-transform infrared (FTIR) spectroscopy, it is difficult to analyse sample solutions with low concentrations of 0.01 to 0.1%, which are common in protein research. For such samples, transmission measurements are challenging, and the sensitivity of conventional ATR methods is insufficient for acquiring high-quality spectra. To address these problems, we have developed a new multi-reflection high-sensitivity ATR unit.

JASCO FTIR spectrometer
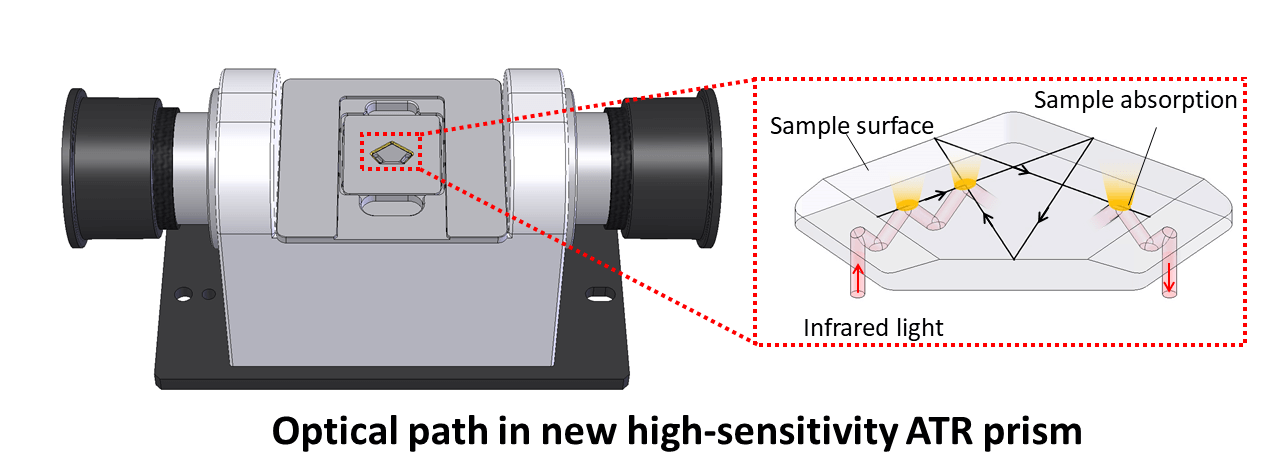
High-sensitivity ATR prism
We developed a high-sensitivity ATR prism involving 14 optical reflections for installation inside the FTIR sample chamber. The optical path in the prism is shown below.
| Prism material | Ge |
| Number of reflections | 14 |
| Incidence angle | 45º |
| Detector | MCT-PV |
Experimental
Comparison of high-sensitivity ATR and single-reflection ATR
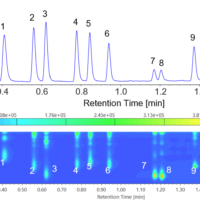
Difference spectra* for a 0.1% lysozyme aqueous solution measured by high-sensitivity ATR and single-reflection ATR are shown below. For ease of comparison, the single-reflection spectrum is magnified by a factor of 14.
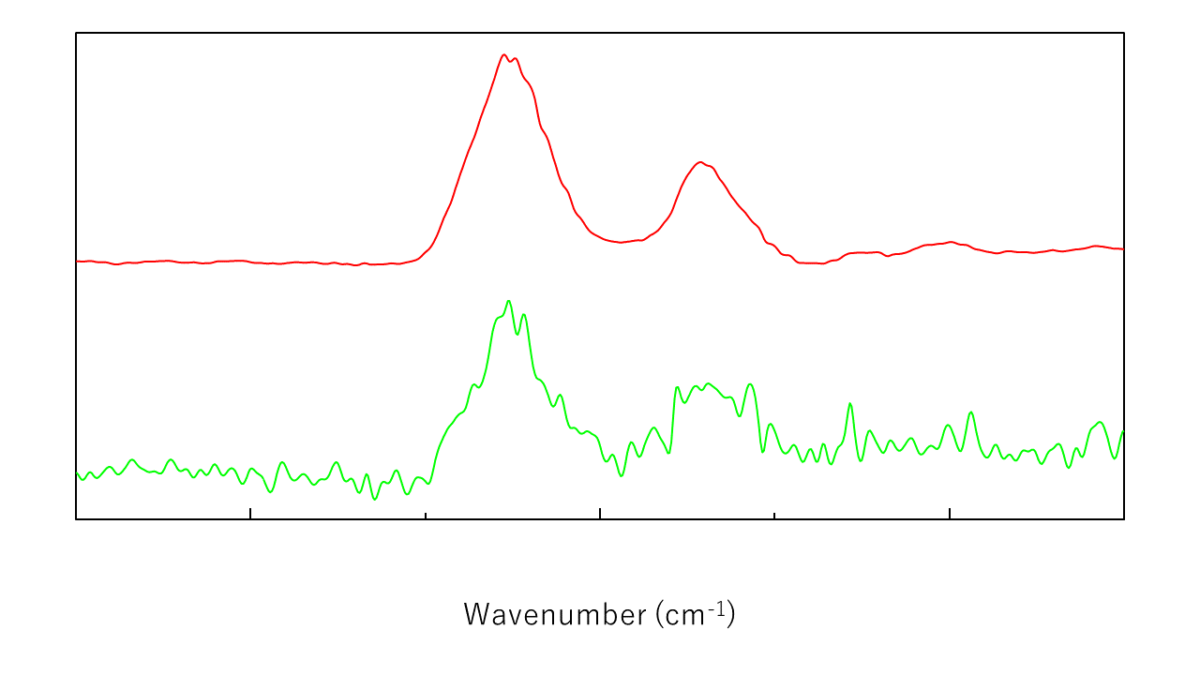
Difference spectra for lysozyme aqueous solution measured using high-sensitivity ATR and single-reflection ATR
Lower limits for detection and quantification of sucrose aqueous solution
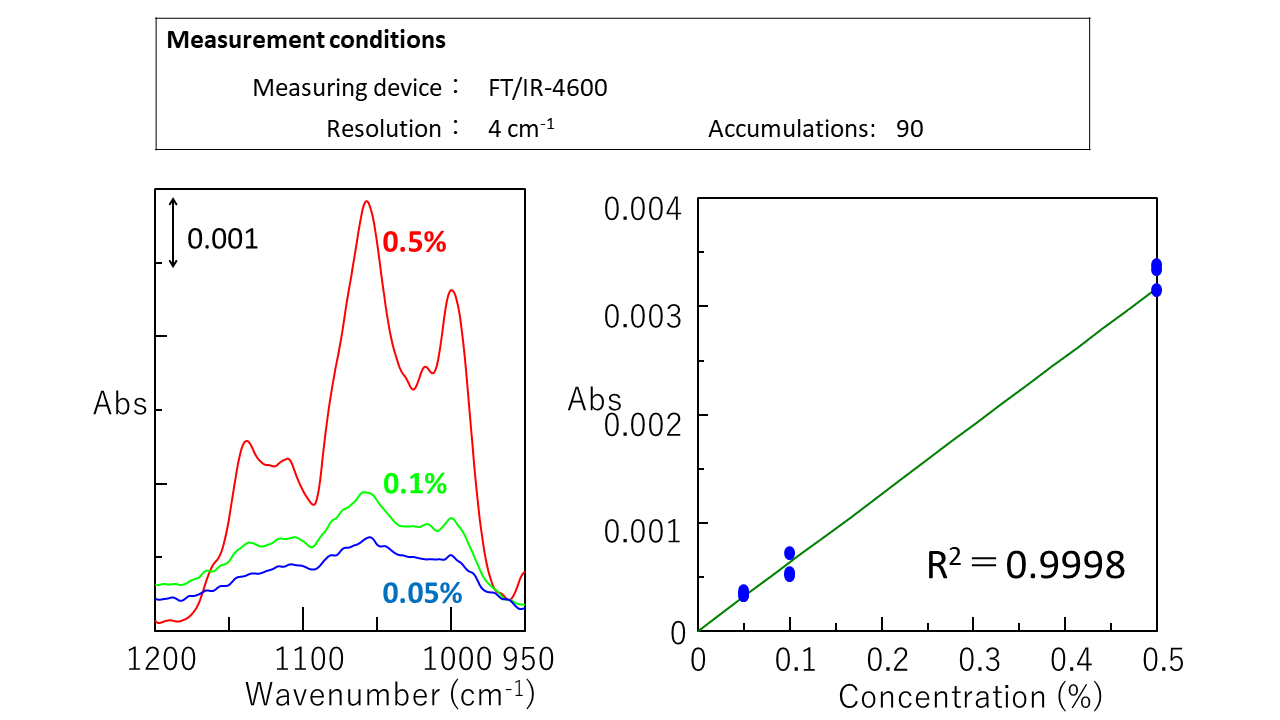
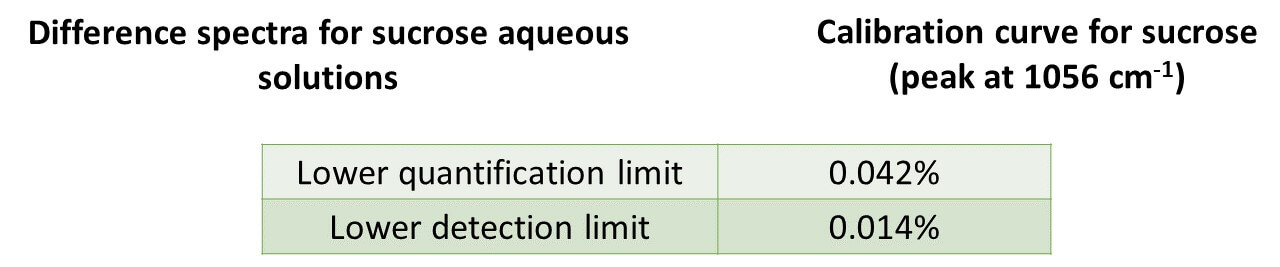
Difference spectra* for 0.05%, 0.1%, and 0.5% sucrose aqueous solutions are shown below, together with a calibration curve based on the peak at 1056 cm-1. It can be seen that there is a good correlation between the peak height and the solution concentration. The calculated lower detection limit (3s) and lower quantification limit (10s) based on the noise level were 0.014% and 0.042%, respectively.
Measurement of lysozyme heavy aqueous solution
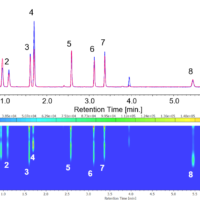
The difference spectrum* for a 0.01% lysozyme aqueous solution and protein secondary structure estimation analysis (SSE) spectra using the amide-I band are shown below. The fractions of α-helix and β-sheet components were calculated to be 40% and 22%, respectively, which are in reasonable agreement with the values of 36% and 10% obtained by Sarver et al.1)
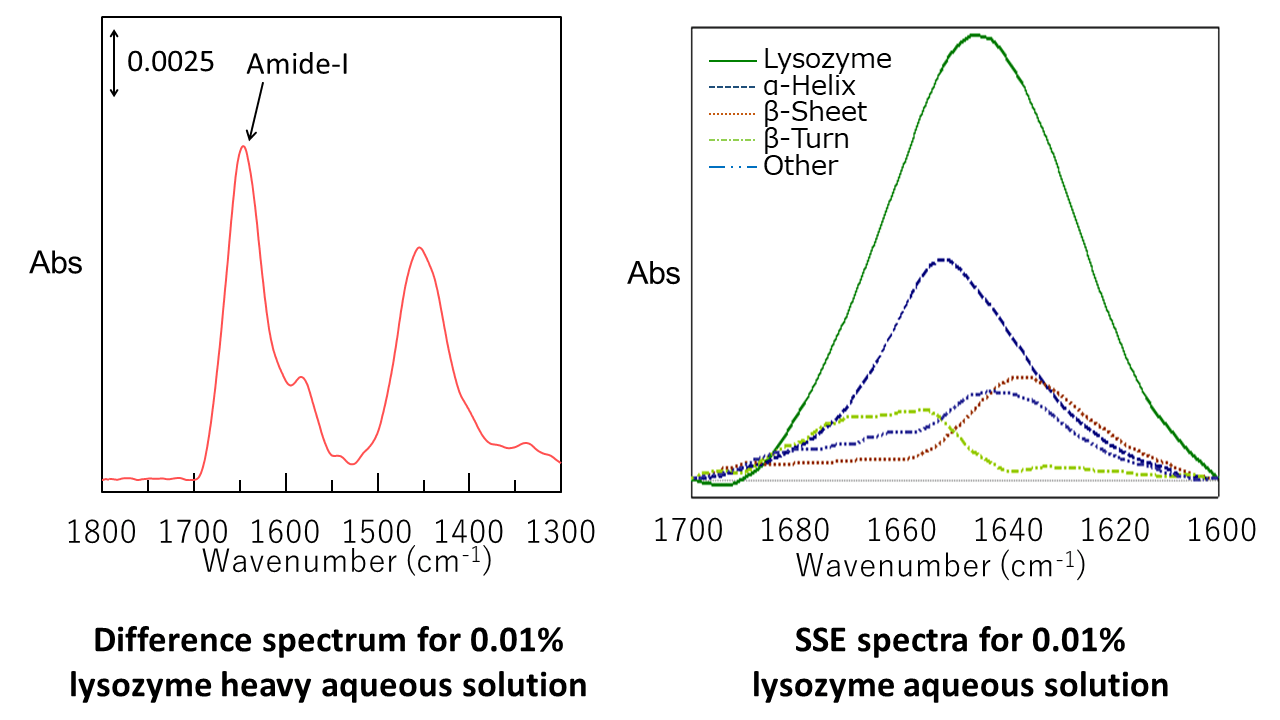
| Structure | Fraction (%) |
|---|---|
| α-Helix | 40 |
| β-Sheet | 22 |
| β-Turn | 18 |
| Other | 20 |
Conclusion
・We developed a high-sensitivity ATR prism.
・For a sucrose aqueous solution, a calibration curve with good linearity was obtained, and the detection limit was found to be 0.014%.
・Detection of lysozyme at a concentration of 0.01% was possible, and an SSE analysis was performed based on the Amide-I band in the difference spectrum.
The newly developed high-sensitivity ATR prism is a promising tool in biochemistry fields such as lipids, saccharides and proteins.
* Calculated by subtracting the solvent spectrum from the aqueous solution spectrum.
References
[1] Sarver, R. W., Krueger, W. C., 1991. Anal. Biochem., 194, 89-100.
Author
Higuchi, Yuji; Kanno, Miyuki; Onishi, Inori; Ito, Chisato; Watanabe, Keisuke
JASCO Corporation
This application was introduced at ICAVS 10 in New Zealand.