Introduction
Supercritical fluid chromatography (SFC) uses carbon dioxide as the main solvent in the mobile phase and is characterized by its ability to maintain high separation efficiency even when performing analyses at high flow rates (linear velocity). Among the sample components that are separated and fractionated by taking advantage of this feature are chiral compounds. To investigate the optimal separation conditions for chiral compounds, a screening method is used to comprehensively analyze individual substances using solvents and columns. Recently, chiral columns with a 3 µm particle size have been developed for SFC, and are expected to provide faster and more efficient chiral-compound separation than conventional SFC using 5 µm particle-size columns.
In this application note, we used a JASCO SFC system with the Method Scouting Support Program, an add-in software for ChromNAV Ver. 2, and performed detection using a photodiode array (PDA) detector.Three modifier solvents and 10 chiral columns with a 3 µm particle size for high speed and high efficiency were used for fast screening of flurbiprofen and ketoprofen, which are acidic non-steroidal anti-inflammatory drugs with anti-inflammatory and analgesic activities. As a result, we report that we were able to find conditions under which good separation was obtained in the chiral separation of acidic compounds without the use of additives such as trifluoroacetic acid.

SFC method scouting system
Experimental
Instruments
CO2 pump: PU-4380
Modifier pump: PU-4185*
Autosampler: AS-4350
Column oven: CO-4065*
PDA detector: MD-4010*
BP regulator: BP-4340
* with option units
SFC Conditions
Column: CHIRALPAK IA/SFC, IB-N/SFC, IC/SFC, ID/SFC, IE/SFC, IF/SFC, IG/SFC, IH/SFC, IJ/SFC, IK/SFC (3.0 mmI.D. x 50 mmL, 3 µm)※
Eluent : Carbon dioxide / modifier (75/25)
Modifier: A; Methanol B; Acetonitrile / ethanol (80/20) C; t-Butyl methyl ether / ethanol (80/20)
Flow rate: 1.2 mL/min
Column temp.: 40 ºC
Wavelength: 210 nm (MD-4010)
Back pressure: 15 MPa
Inj. volume: 1 µL
Sample: 0.1 mg/mL Flurbiprofen in methanol, 0.5 mg/mL Ketoprofen in methanol
* CHIRALPAK is a trademark or registered mark of Daicel Corporation.
Structures

Schematic Diagram
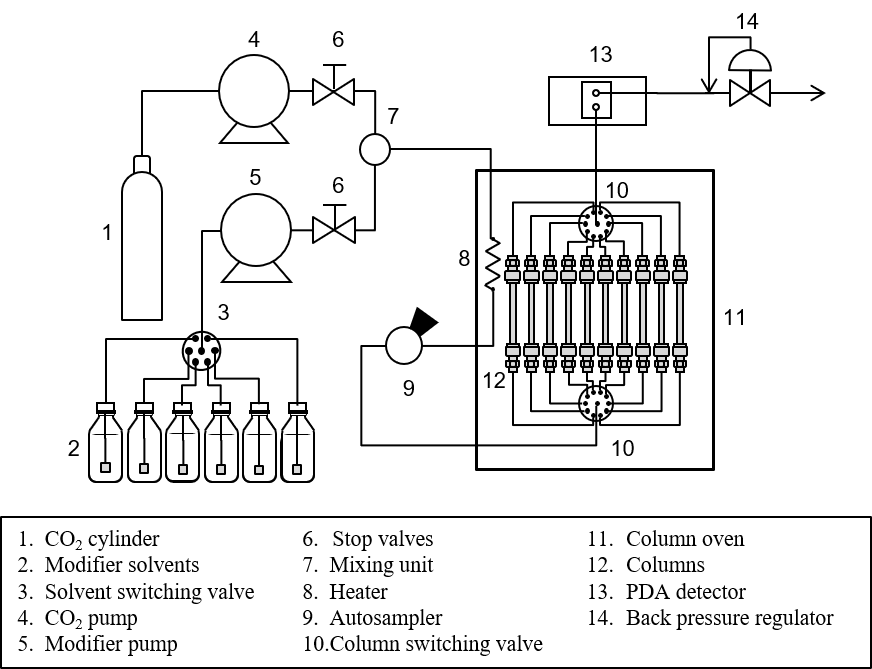
Keywords
Flurbiprofen, Ketoprofen, SFC, screening, method scouting, chiral separation, CD detector, CHIRALPAK, 3 µm
Results
The column stabilization time during screening was set to 10 min only for the first column after solvent changeover and 3 min for the other columns, taking into account solvent substitution in the system. The analysis time was set to 3.0 min. The total required time for this screening was 3.4 hours per compound.
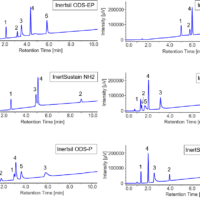
Figure 1 and Figure 2 show the screening results for flurbiprofen and ketoprofen, respectively. Each row of chromatograms represent a different modifier solvent, and each column of chromatograms represents a different chiral column.
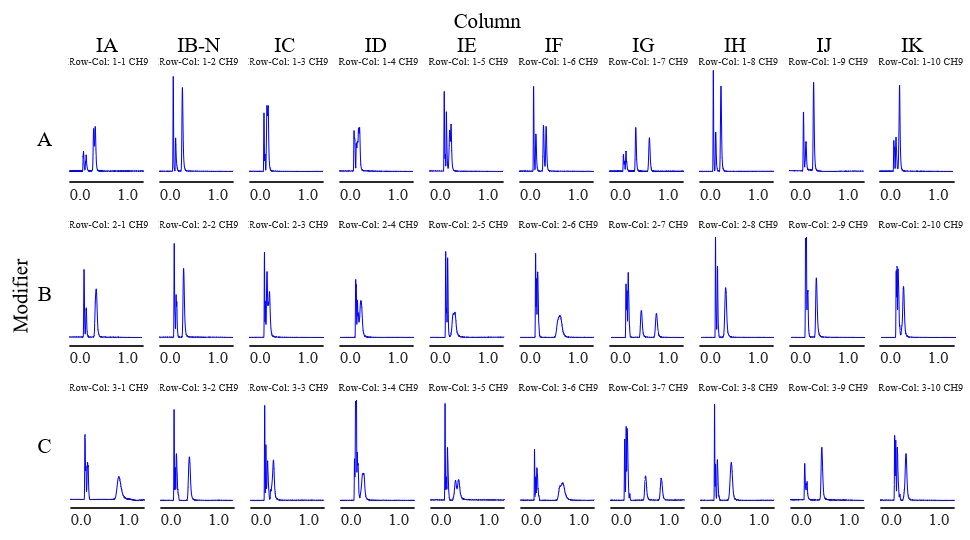
Fig. 1 Chromatograms of flurbiprofen obtained using method scouting system (PDA detection wavelength: 210 nm)
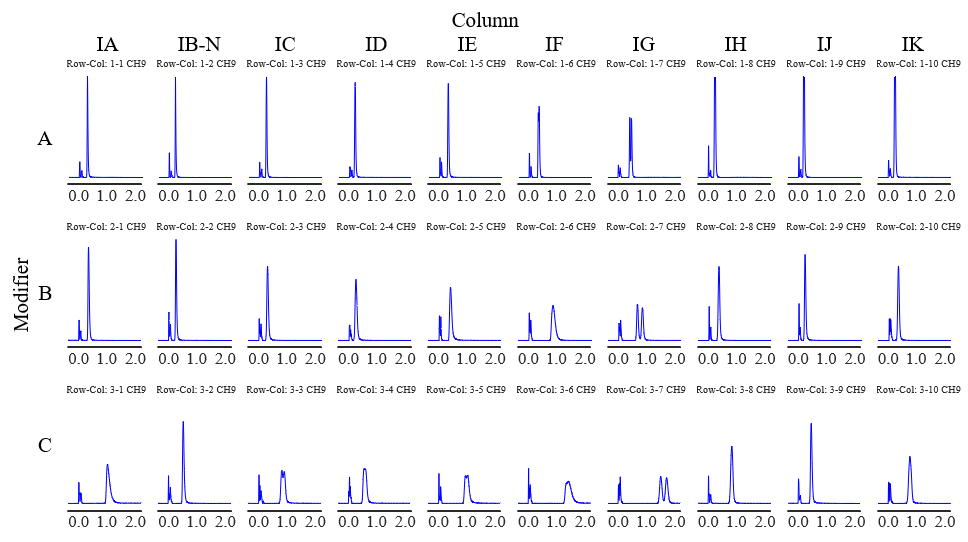
Fig. 2 Chromatograms of ketoprofen obtained using method scouting system (PDA detection wavelength: 210 nm)
Tables 1 and 2 show the degree of separation of flurbiprofen and ketoprofen under each condition. Results with a resolution of 1 or less are marked as incomplete separation (I.S.). Good separation was obtained for both compounds using CHIRALPAK IG/SFC as the column. In contrast, incomplete separation was observed at the peak apex when using CHIRALPAK IA, IC, ID, IE, and IF.
In addition, although additives such as phosphoric acid and trifluoroacetic acid are usually used for chiral separation of acidic compounds in HPLC, good separation was obtained without any additives for chiral separation by SFC. This is believed to be due to the weak acidity of supercritical carbon dioxide.
Table 1 Comparison of flurbiprofen resolution
| Column | |||||||||||
| IA | IB-N | IC | ID | IE | IF | IG | IH | IJ | IK | ||
| Modifier | Methanol | I.S. | N.S. | N.S. | N.S. | N.S. | 1.41 | 4.90 | N.S. | N.S. | N.S. |
| Acetonitrile / ethanol | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | 4.25 | N.S. | N.S. | N.S. | |
| t-Butyl methyl ether / ethanol | N.S. | N.S. | N.S. | N.S. | I.S. | N.S. | 4.27 | N.S. | N.S. | N.S. | |
I.S.: Incomplete Separation, N.S.: Not Separated
Table 2 Comparison of ketoprofen resolution
| Column | |||||||||||
| IA | IB-N | IC | ID | IE | IF | IG | IH | IJ | IK | ||
| Modifier | Methanol | N.S. | N.S. | N.S. | N.S. | N.S. | I.S. | 1.20 | N.S. | N.S. | N.S. |
| Acetonitrile / ethanol | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | 1.49 | N.S. | N.S. | N.S. | |
| t-Butyl methyl ether / ethanol | N.S. | N.S. | I.S. | I.S. | I.S. | N.S. | 1.30 | N.S. | N.S. | N.S. | |
I.S.: Incomplete Separation, N.S.: Not Separated
Conclusion
In this study, the use of a size-reduced chiral column with 3 µm particle size in SFC enabled rapid method scouting, allowing the selection of separation conditions to be completed within just 3.4 hours. Conditions that resulted in only peak apex separation have the potential to be further optimized to achieve baseline separation by adjusting the column dimensions and modifier.
In chiral separation by SFC, we were able to find conditions under which good separation could be obtained for chiral separation of acidic compounds without using additives such as trifluoroacetic acid.
The SFC method scouting system can be effectively used to explore separation conditions for chiral compounds because SFC allows separation studies in a shorter time compared to HPLC.