Introduction
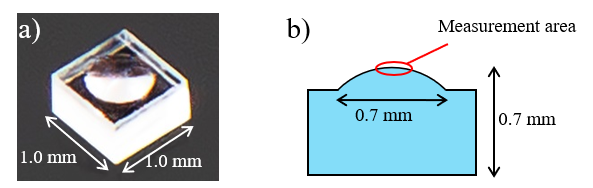
The use of near-infrared (NIR) spectroscopy in the pharmaceutical industry has grown rapidly in the past decade. Infrared microscope system which is integrated with a NIR capable FTIR instrument enables rapid imaging of pharmaceutical tablets, and provides the valuable information for the quality control of tablet properties. This article shows the evaluation example of pharmaceutical tablet (Figure 1) by two imaging analysis method.
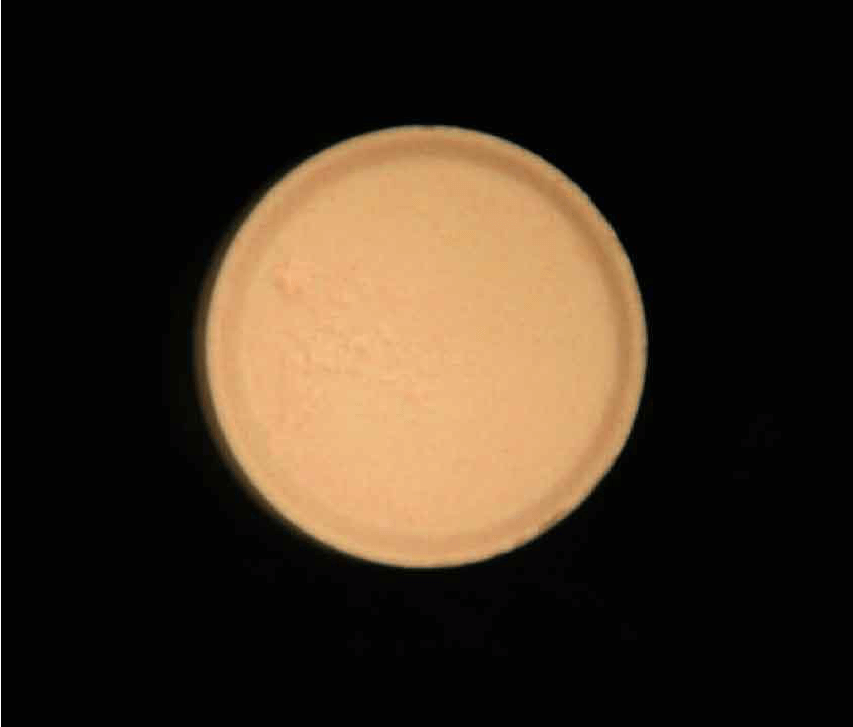
Figure 1. Observation view
Figure 2 shows the result of imaging analysis by peak height. Imaging by peak height enables to find the location of Raman activity easily.
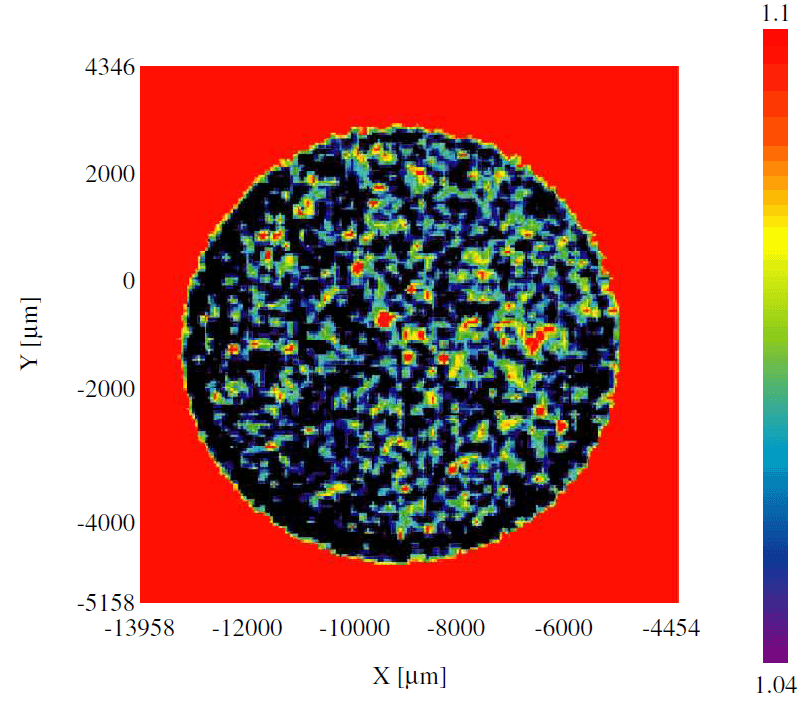
Figure 2. Imaging view by peak height (1935 nm)
Figure 3 shows the imaging result of Principal Component Analysis (PCA). PCA can classify the multi-component samples, and enables to evaluate the component distribution and the contaminant identification.
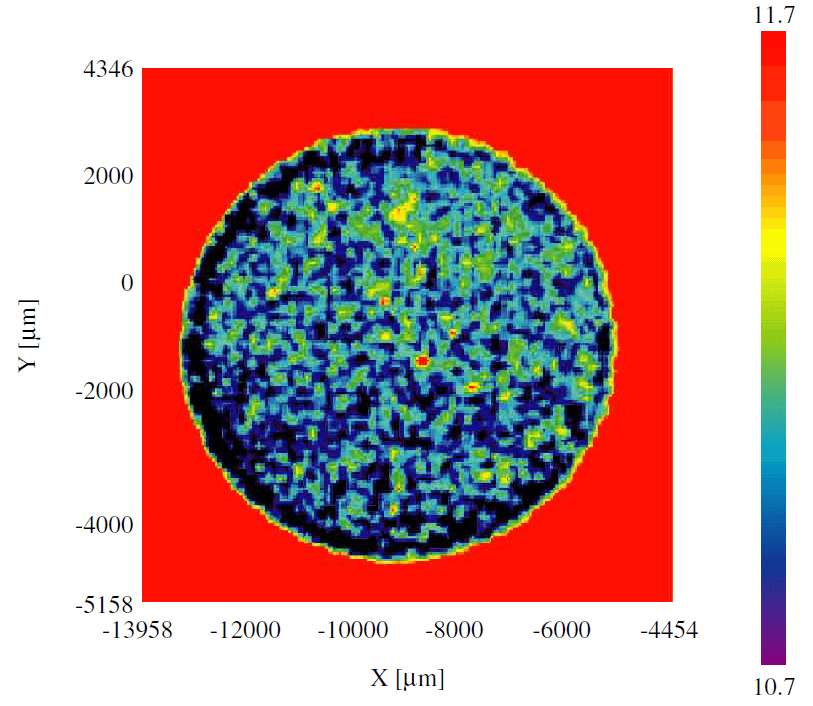
Figure 3. PCA mapping
The principal component analysis can be a useful tool because specific peaks contained in the data can be extracted and the compositional distribution is simply analyzed by the component grouping, even for measurement results that are seemingly difficult to identify from the individual component spectra.