Introduction
Cloperastine fendizoate is a drug newly listed in the 18th edition of the Japanese Pharmacopoeia (JP), and is used to relieve respiratory symptoms such as coughing by directly acting on the cough center.
In this report, we present the results of system suitability tests for purity of cloperastine fendizoate in accordance with the Japanese Pharmacopoeia, 18th Edition.

LC-4500 series HPLC system
Experimental
Instruments
Pump: PU-4580
Degassing unit: DG-4580
Low pressure gradient unit: LG-4580
Autosampler: AS-4550
Column oven: CO-4060
UV detector: UV-4570
Conditions
Column: Unifinepak C18 (4.6 mmI.D. x 150 mmL, 5 µm)
Eluent A: 0.1 M Potassium dihydrogen phosphate/acetonitrile/perchloric acid (400/320/1)
Eluent B: Acetonitrile/0.1 M potassium dihydrogen phosphate/perchloric acid (1050/450/1)
Gradient: A/B = 100/0 (0.0 min) -> 100/0 (12.0 min) -> 0/100 (22.0 min) -> 0/100 (27.0 min) -> 100/0 (27.1 min), 1cycle; 37 min
Flow rate: 1.2 mL/min
Column temp.: 25 ºC
Wavelength: 226 nm
Injection volume: 20 µL
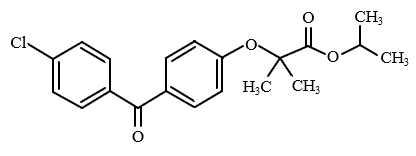
Standard: 4-Cholobenzophenone (The dissolving and diluting solvent is eluent A.)
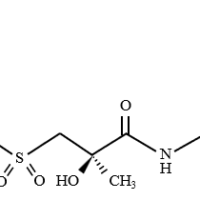
Structure
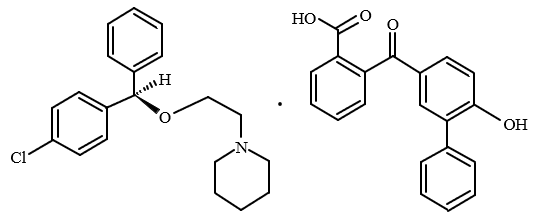
Cloperastine fendizoate
Keywords
Japanese Pharmacopoeia, cloperastine fendizoate, UV detector
Results
An overview of the system suitability tests for the purity of cloperastine fendizoate is shown in Table 1.
Table 1 Overview of system suitability tests for the purity of cloperastine fendizoate
| Test | Criteria |
| Detectability | When the standard solution (1.25 mg/L) is diluted to 0.25 mg/L and the test is performed under the above operating conditions on 20 µL of this solution, the area of the peak associated with 4-chlorobenzophenone is 14–26 % of that of the standard solution. |
| System performance | When the test is performed on 20 µL of the standard solution under the above operating conditions, the number of theoretical plates and the 4-chlorobenzophenone peak symmetry factor are ≥10000 and ≤2.0, respectively. |
| System reproducibility | When the test is repeated 6 times with 20 µL of the standard solution under the above operating conditions, the relative standard deviation of the 4-chlorobenzophenone peak area is ≤2.0 %. |
Figure 1 shows the results of the purity detectability test. The ratio of the 4-chlorobenzophenone peak area for the diluted solution (0.25 mg/L) to that for the standard solution (1.25 mg/L) was 19.5 %, which met the criterion of 14–26 %.
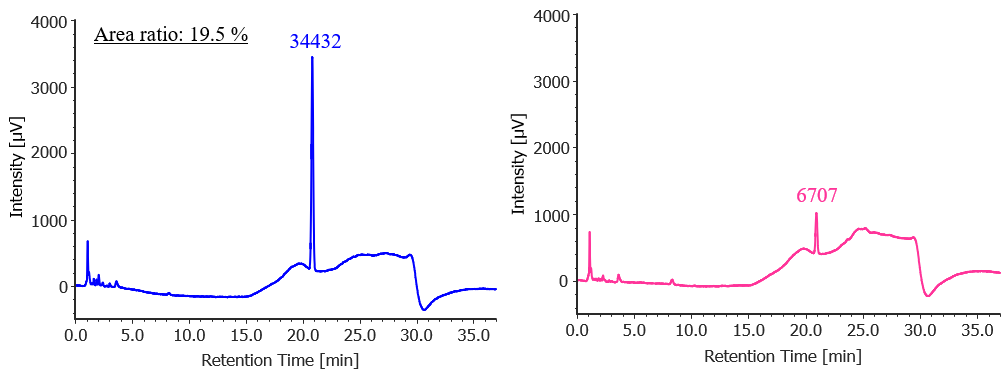
Fig. 1 Chromatograms of 4-chlorobenzophenone for the test of the detectability (left: 1.25 mg/L, right: 0.25 mg/L)
Figure 2 shows chromatograms of 4-chlorobenzophenone standard solutions (n = 6), and Table 2 shows the results for peak area reproducibility based on the measured system performance and system reproducibility. The number of theoretical plates was 80288 (criterion: ≥10000), the symmetry factor was 1.01 (criterion: ≤2.0), and the relative standard deviation of the peak area was 0.21 % (criterion: ≤2.0 %), all of which met the specified criteria.
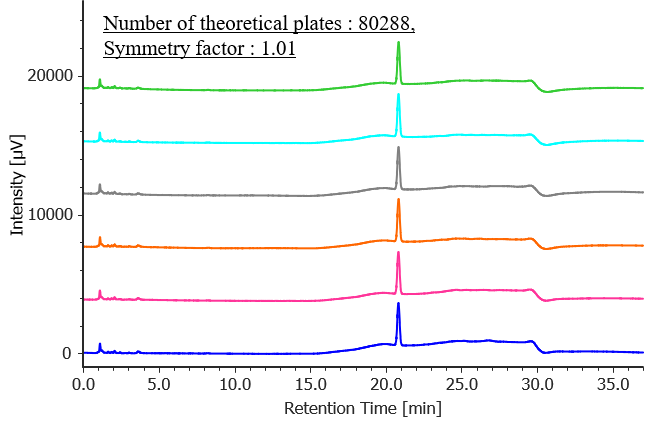
Fig. 2 Chromatogram of 4-chlorobenzophenone standard solution (1.25 mg/L, n = 6)
Table 2 4-Chlorobenzophenone peak area reproducibility (n = 6)
| Injection number | Peak area |
| 1 | 33336 |
| 2 | 33528 |
| 3 | 33513 |
| 4 | 33485 |
| 5 | 33450 |
| 6 | 33443 |
| Ave. | 33459 |
| SD | 69 |
| RSD[%] | 0.21 |
Conclusion
We performed system suitability tests for the purity of cloperastine fendizoate, which is newly listed in the 18th edition of the Japanese Pharmacopoeia. As shown in Table 3, all of the evaluation results met the specified criteria for the Japanese Pharmacopoeia.
Table 3 Content and results of system suitability tests for the purity of cloperastine fendizoate
| Test | Test item | Criterion | Result | Judgement |
| Detectability | Area ratio | 14-26 % | 19.5 % | Passed |
| System performance | Number of theoretical plates | ≥10000 | 80288 | Passed |
| Symmetry factor | ≤2.0 | 1.01 | Passed | |
| System reproducibility | Relative standard deviation of peak area | ≤2.0 % | 0.21 % | Passed |