Introduction
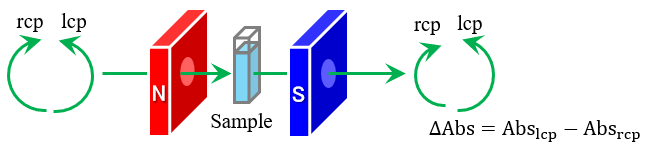
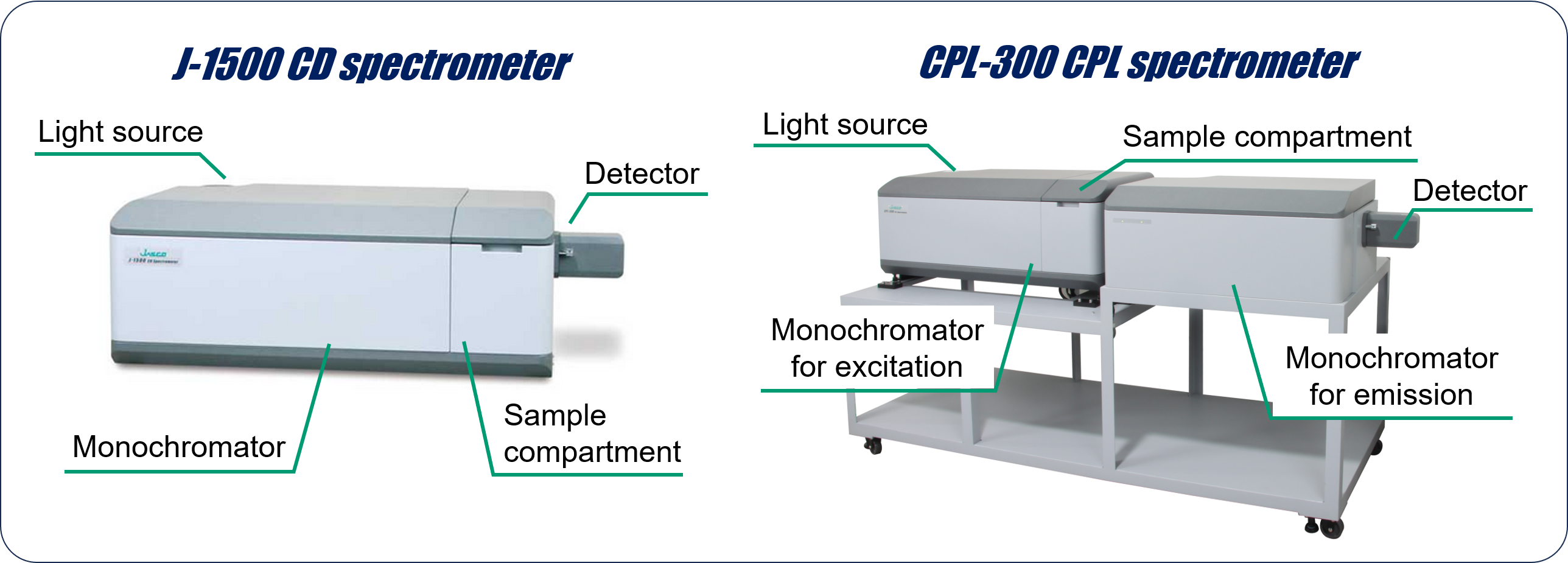
Magnetic circular dichroism (MCD) spectroscopy is a method to measure the difference in absorption of left-handed and right-handed circularly polarized light induced by a magnetic field. MCD spectroscopy has been commonly used to comprehensively evaluate the electronic structure and magnetic and optical properties in the absorption process of a molecule that exhibits photophysical properties, such as phthalocyanines and porphyrins with various central metals and substituents. In these evaluations, a robust, automated, high-throughput measurement technique was considered essential to improve measurement performance as well as to perform efficient and comprehensive measurements. Therefore, we developed a high-throughput MCD (HTMCD) system that can measure MCD and absorption spectra of up to 120 samples by automating sample aspiration, measurement, and washing. In this presentation, the configuration and basic performance of the HTMCD system are introduced. Using this system, MCD and absorption spectra of phthalocyanine complexes with various central metal ions and substituents were obtained, and the orbital angular momenta of each complex were calculated and the differences between them are discussed.
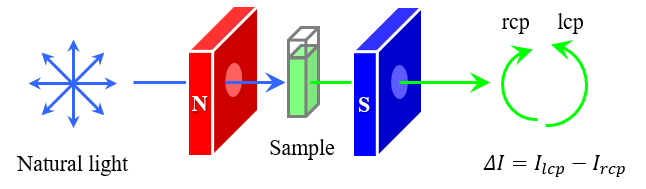
We have also adapted the HT concept to magnetic circularly polarized luminescence (MCPL) spectroscopy, which is used to characterize the electronic structure, magnetic and optical properties during luminescence processes, and constructed the HTMCPL system. For phthalocyanine complexes, the effectiveness of this system is then demonstrated through applications related to solvent effects on gMCPL values and a measurement method that is not affected by photodegradation.
Experimental
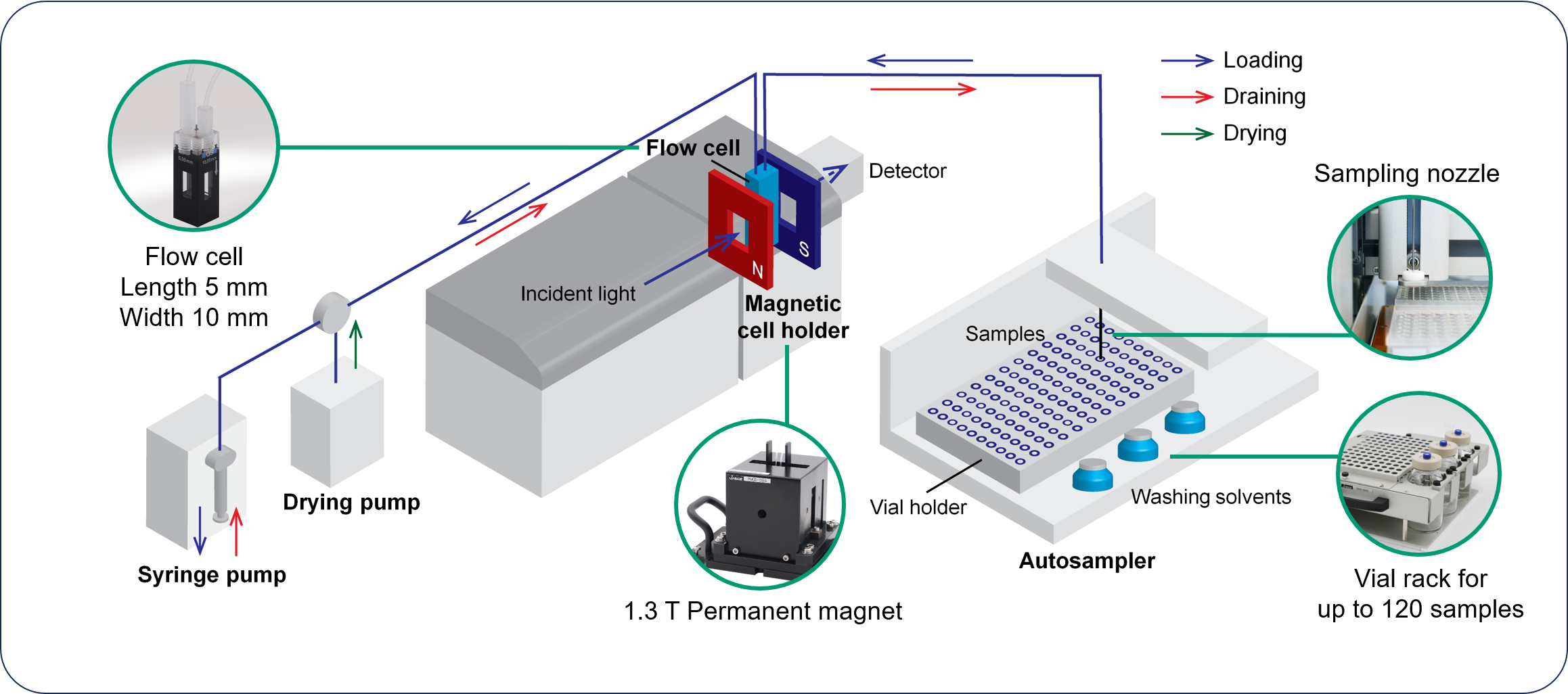
Measurement system
Schematic diagram of HTMCD system
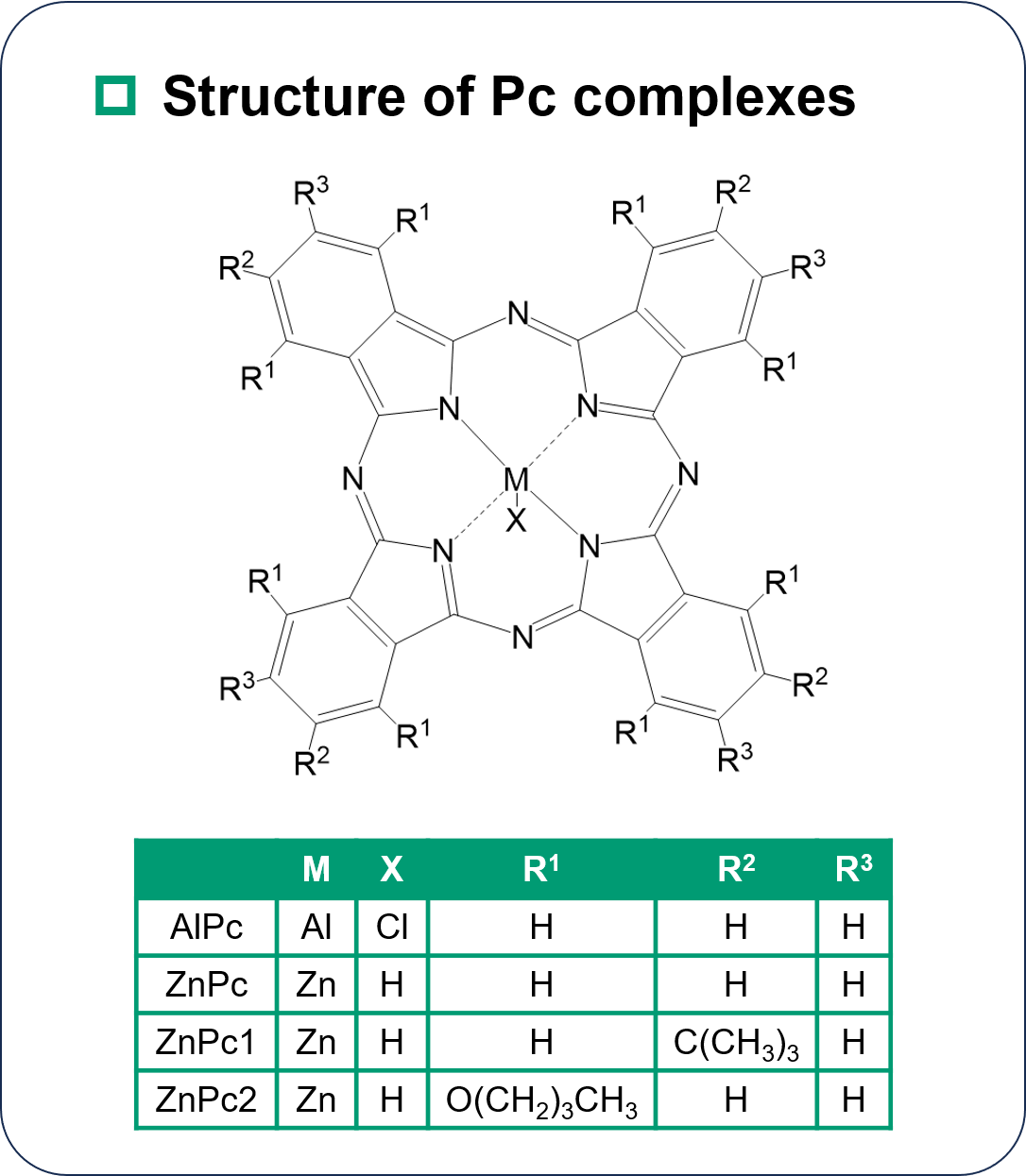
Materials
Results
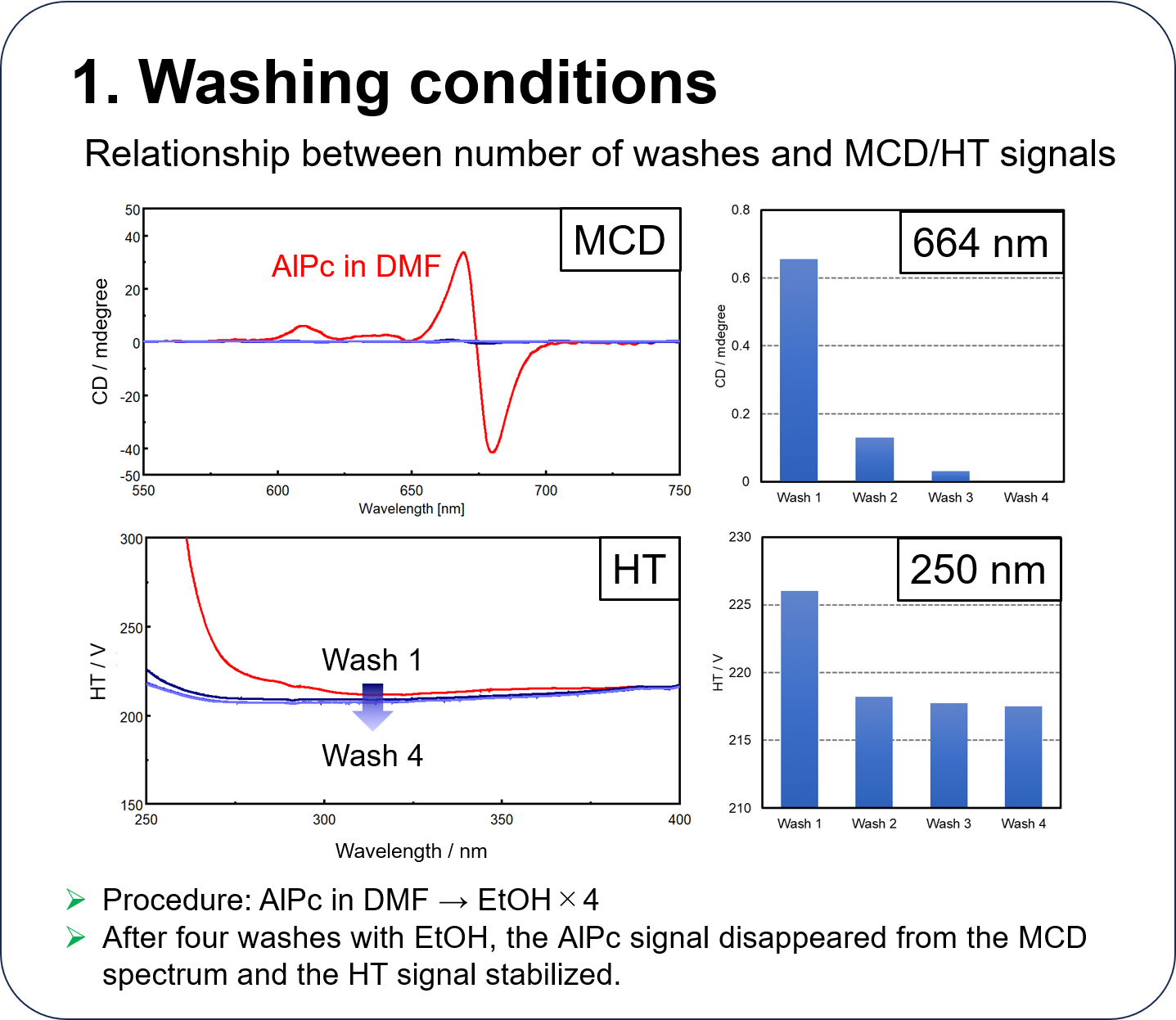
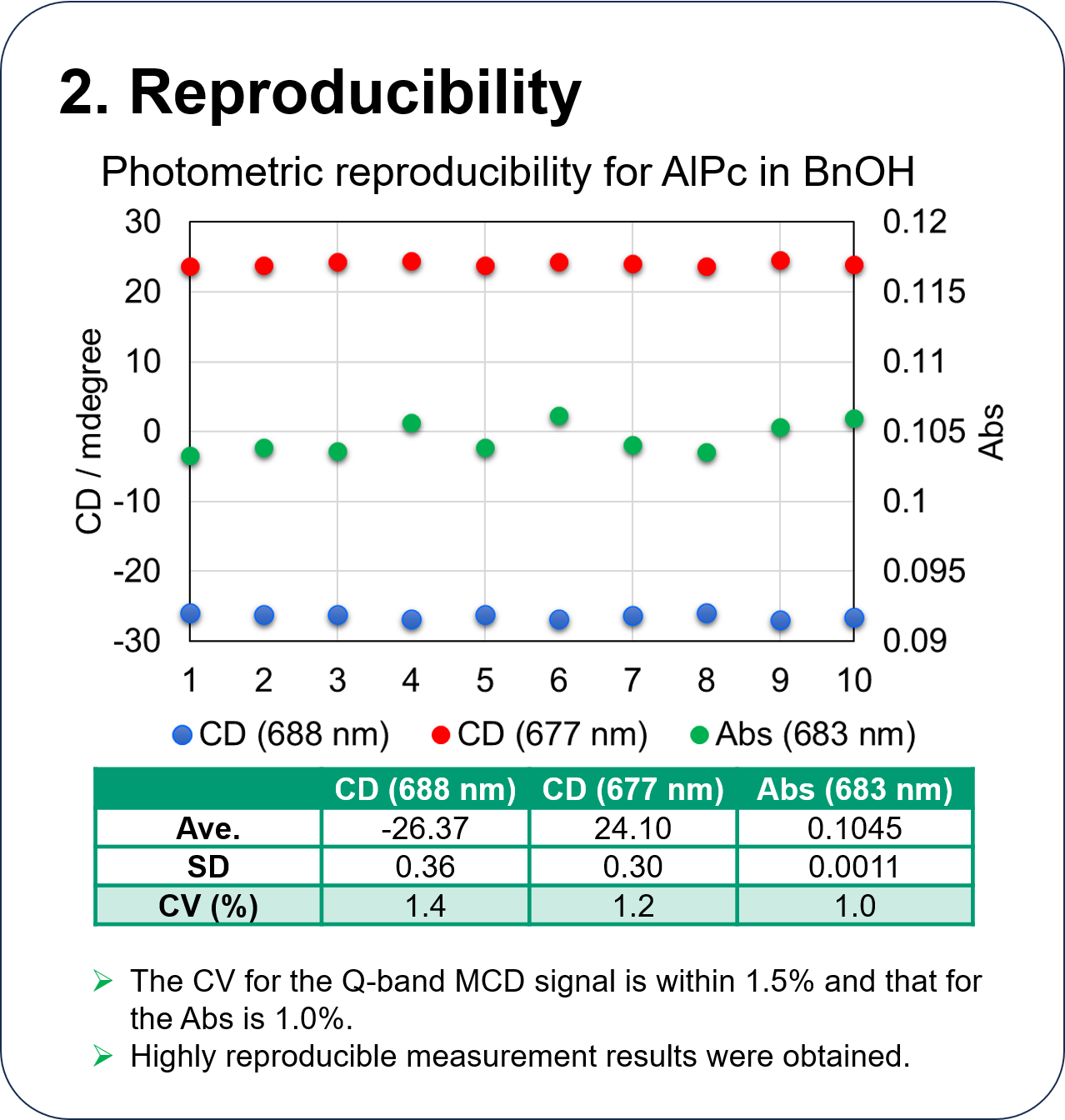
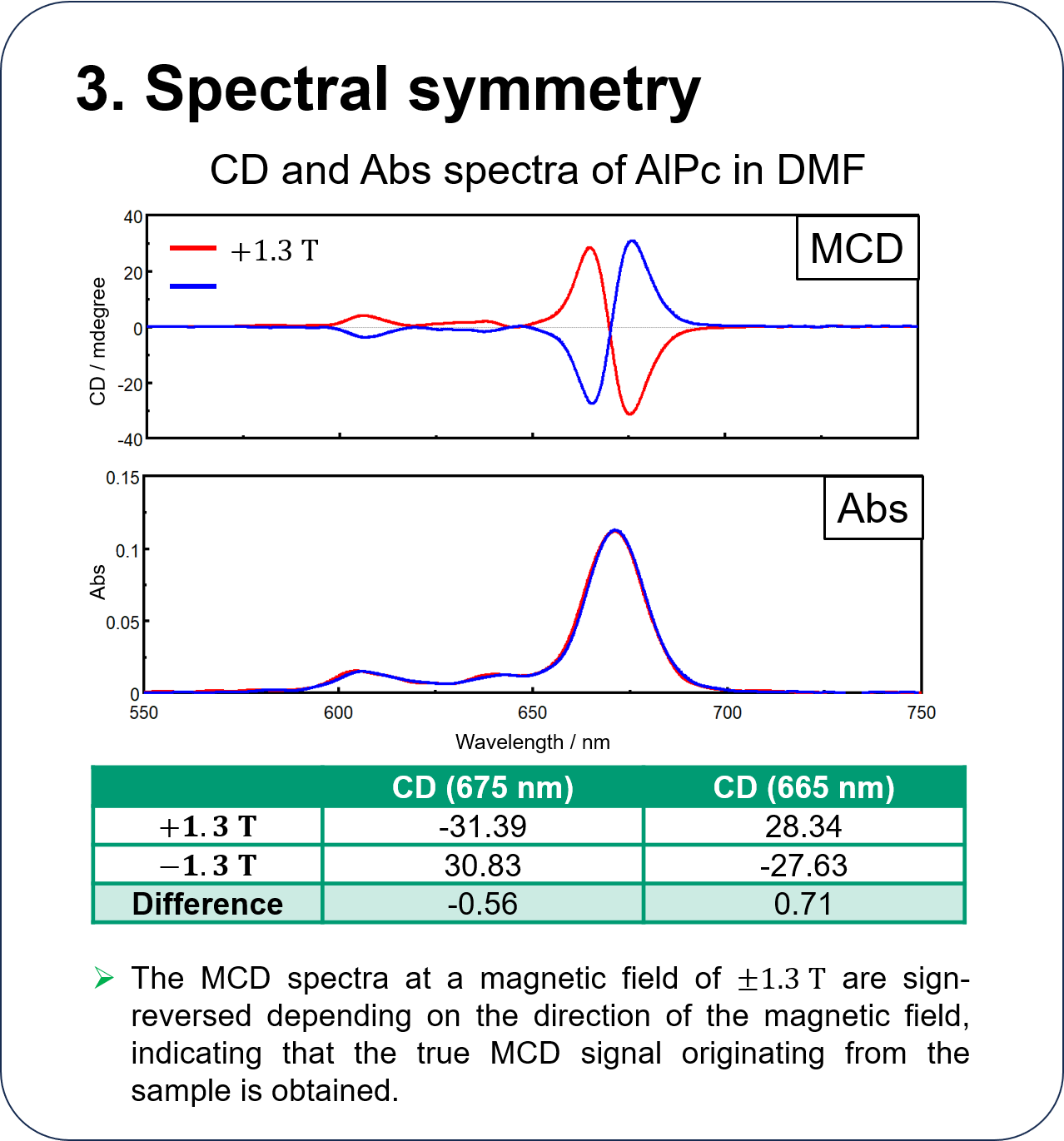
Performance of HTMCD system
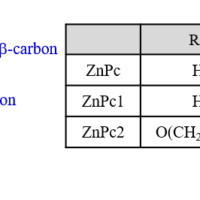
Evaluation of substituent effect on |Lz| during absorption process using HTMCD system
TD-DFT results
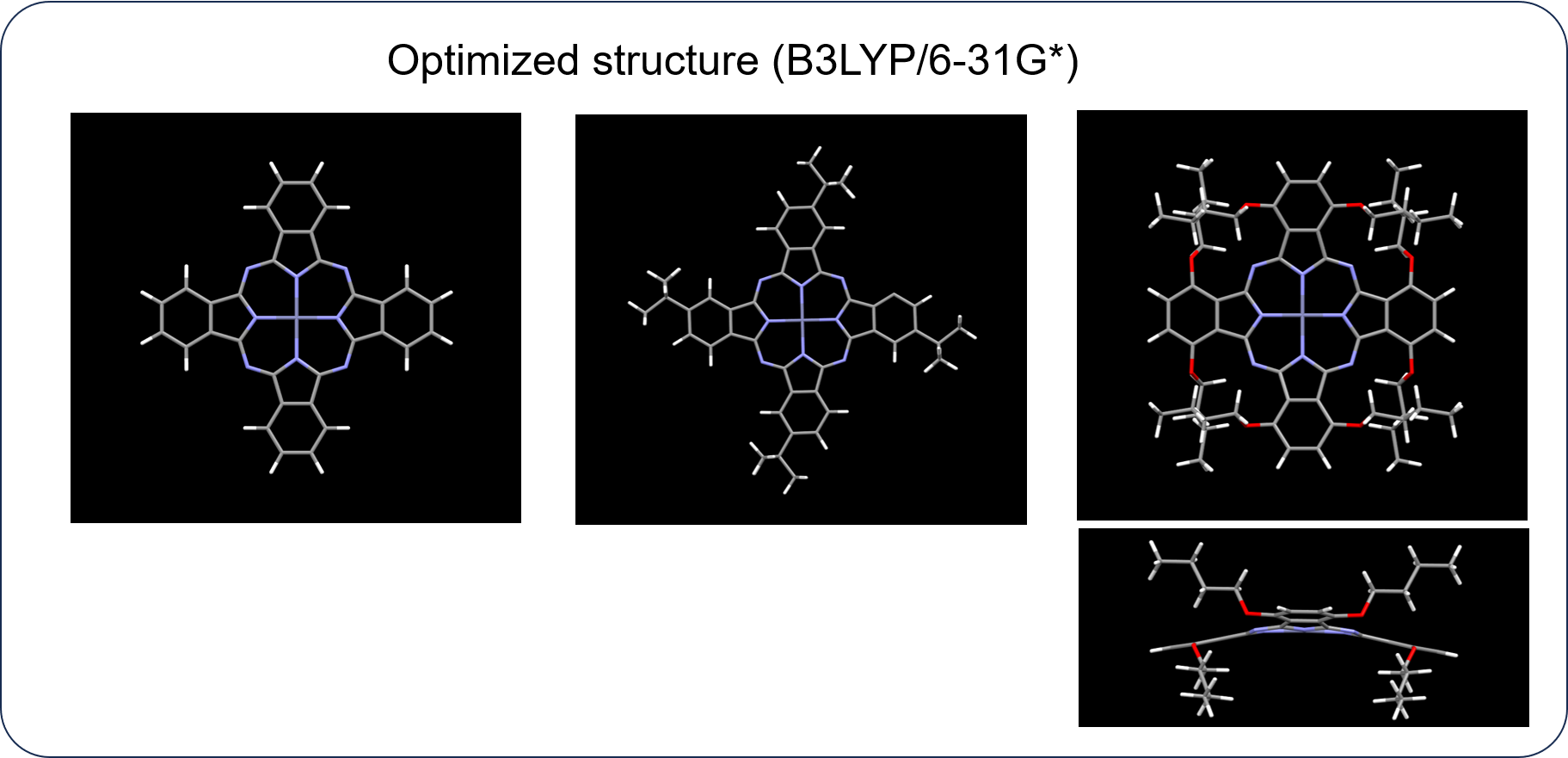
While ZnPc and ZnPc1 maintain the robust planarity of the phthalocyanine skeleton, ZnPc2 exhibits a saddle-shape conformation due to steric hindrance of the substituents.
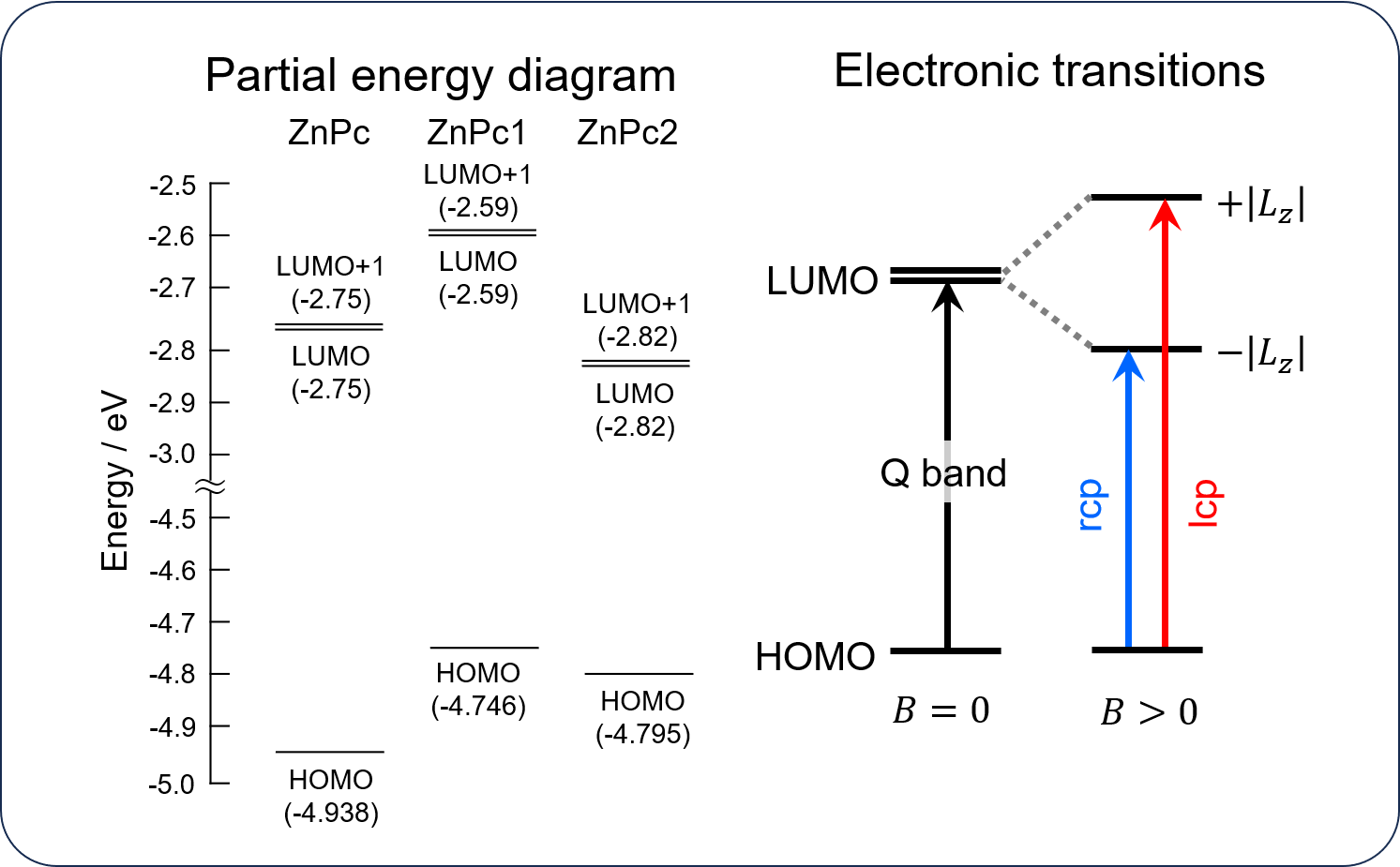
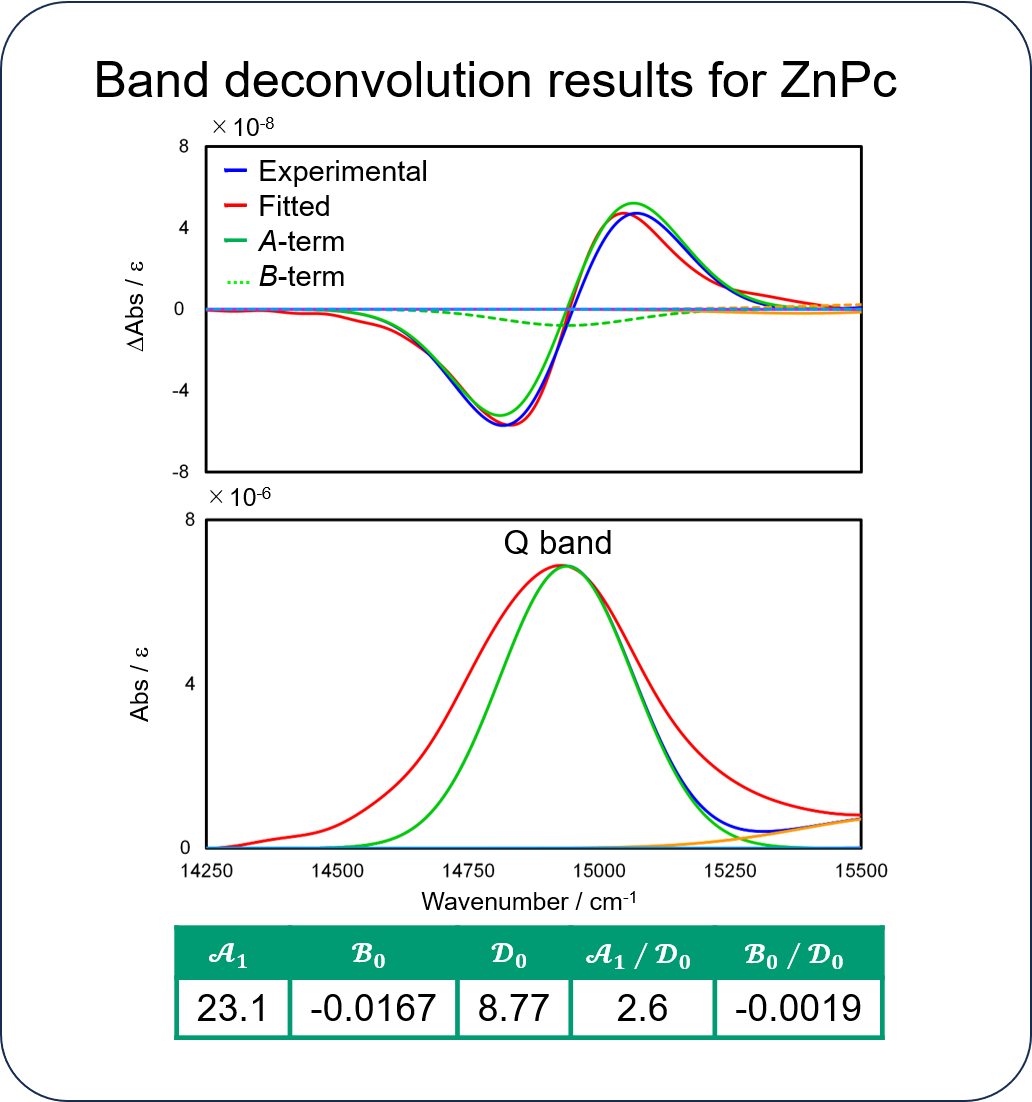
The TD-DFT calculations show that the Pc complexes have doubly degenerate LUMO. When a magnetic field is applied to the molecules, the LUMO splits into two energy levels due to the Zeeman effect, where the lower energy orbital absorbs rcp light and the higher energy orbital absorbs lcp light. As a result, the MCD spectra show a large A-term.
MCD spectrum of ZnPc and orbital angular momentum
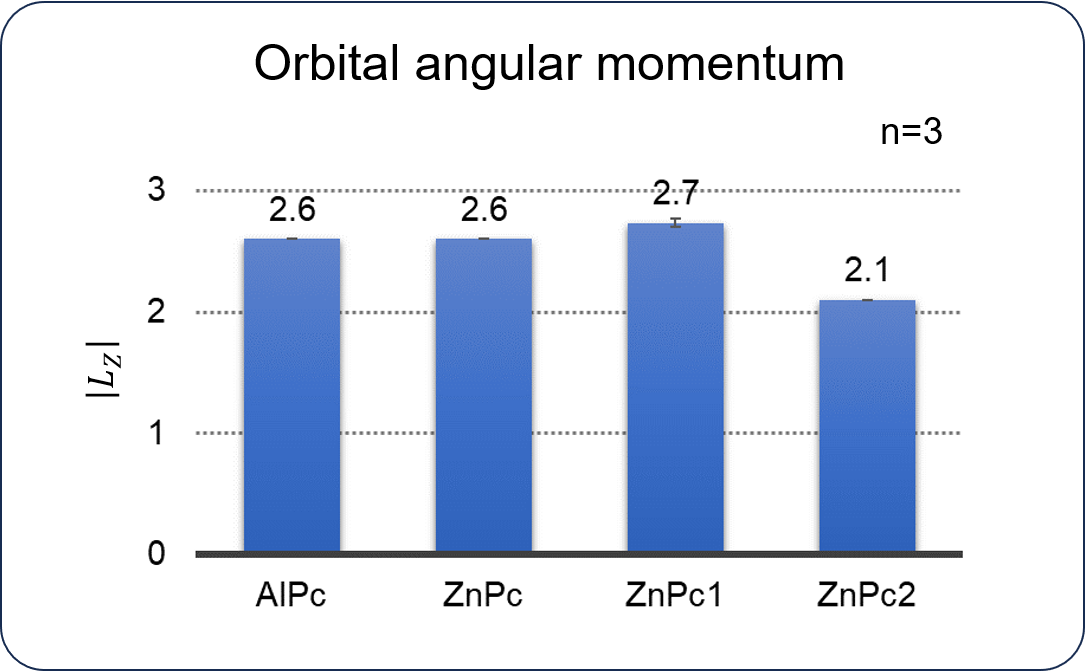
While no significant difference in |Lz | was observed for AlPc, ZnPc, and ZnPc1, ZnPc2 exhibits small |Lz | value.
The difference between ZnPc2 and other complexes is the distortion of the Pc.
Therefore, it is considered that the presence or absence of this distortion affects |Lz |.
Solvent effect on gMCPL values for AlPc
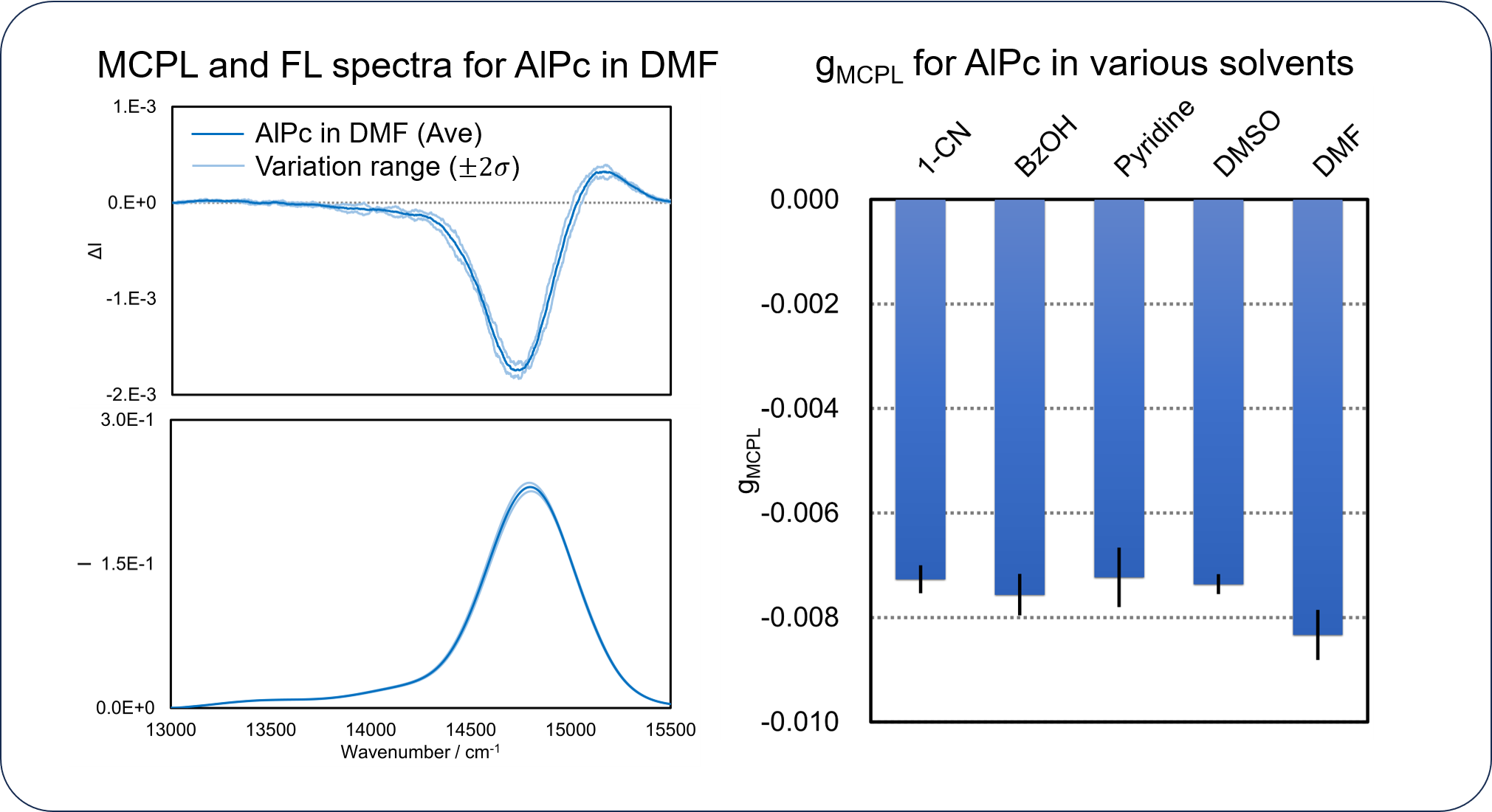
It is important to identify the range of gMCPL variation in order to compare gMCPL values between different samples in a meaningful way, and the HTMCPL system is effective for this measurement.
Using the HTMCPL system, MCPL and FL spectra of AlPc dissolved in five different solvents were measured three separate times to obtain the range of gMCPL variation.
For the MCPL and FL spectra of AlPc solution in DMF, the negative signals at lower wavenumbers are larger than the positive signals at higher wavenumbers, indicating that the lower-energy orbitals are occupied by more electrons in the luminescence process.
A one-way analysis of variance (ANOVA) was performed to determine if gMCPL varies with solvent, and the p-value of 0.00030 is below the significance level of 0.05, suggesting that gMCPL shows significant differences among the five solvent conditions.
Evaluation of photodegradable samples
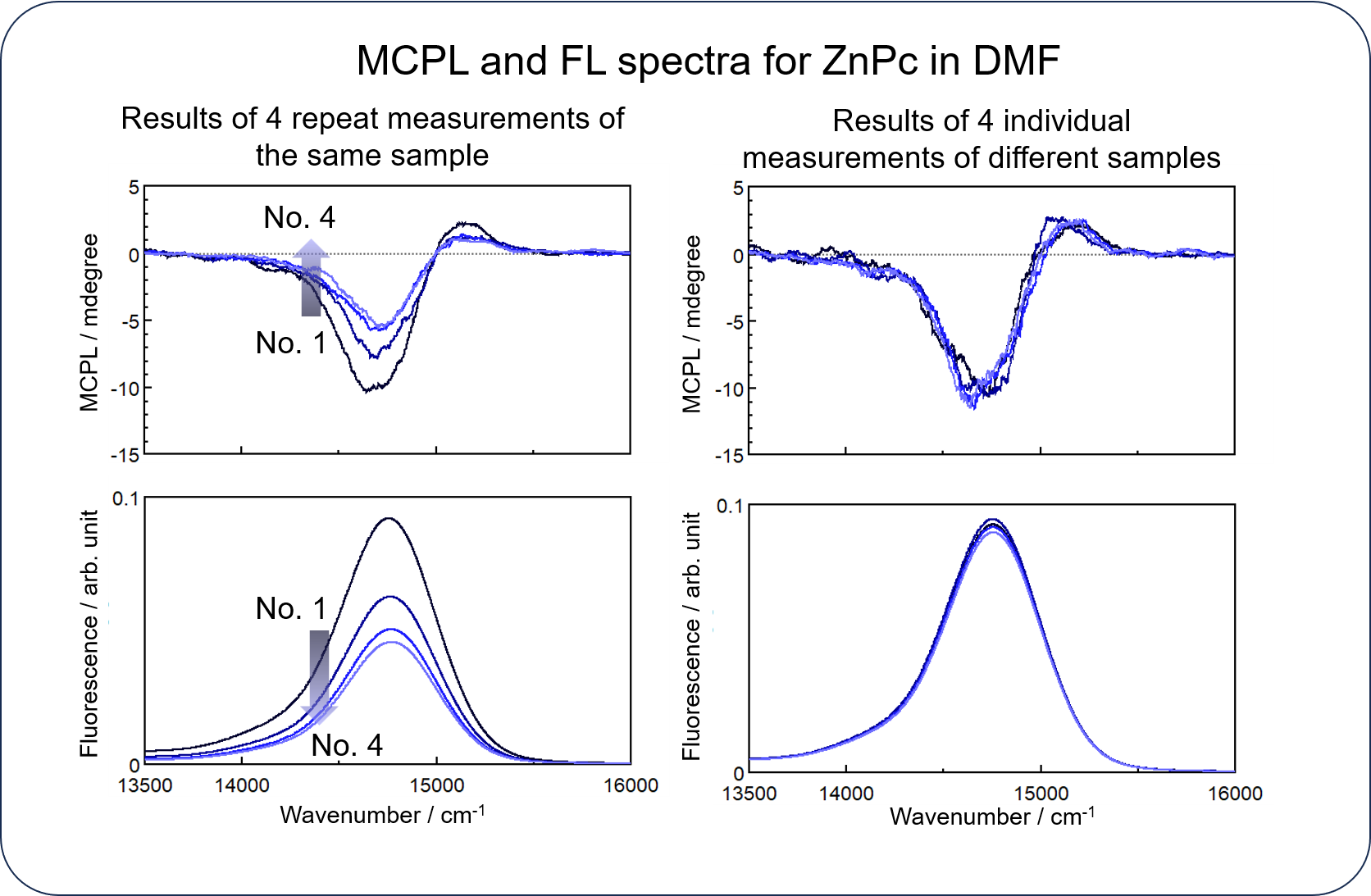
In MCPL measurements, accumulation is commonly performed to improve the signal-to-noise ratio of a spectrum, but the excitation light often causes photodegradation of a sample, resulting in a gradual decrease in the intensity of both spectra when the same sample is repeatedly measured.
The HTMCPL system can easily improve the S/N ratio without being affected by photodegradation by automatically obtaining individual spectra for the same number of samples as the number of accumulations.
Conclusion
The HTMCD and HTMCPL systems, which can automatically measure up to 120 samples, were constructed.
The performance evaluation results for the HTMCD system demonstrated that this system can be used for the study on phthalocyanines and porphyrins.
Through application examples, we have shown that both systems can be utilized to efficiently and comprehensively evaluate the electronic structure, magnetic properties, and asymmetry factors for phthalocyanine complexes under various solvent conditions or with a variety of central metals and substituents.
References
Poster Session at Italia-Japan Binational Conference on Chiroptical and Related Phenomena (January 7 – 8, 2025, in Kitasato University, JAPAN)
Satoko Suzukia, Akio Kanetaa, Anas Santriab, Yoshitane Imaic, Hiroyuki Nishikawad, Ken-ichi Akaoa ,* and Naoto Ishikawab,*
aJASCO Corporation, 2967-5 Ishikawa-machi, Hachioji, Tokyo 192-8537, Japan
bGraduate School of Science, Osaka University, 1-1 Machikaneyama-cho, Toyonaka, Osaka 560-0043, Japan
cFaculty of Science and Engineering, Kindai University, 3-4-4 Kowae, Higashiosaka, Osaka 577-8502, Japan
dFaculty of Science, Ibaraki University, 2-1-1 Bunkyo, Mito, Ibaraki 310-8512, Japan