What is FDA 21 CFR Part 11?
FDA 21 CFR Part 11 is a set of regulations regarding digital archiving of data and data records for GLP, GCP, and GMP procedures. These regulations cover a number of areas, including: 1) access control and electronic signature requirements for data recorded by any computer-controlled analytical system in which the results are digitally archived; 2) the provision of security functions that can only be accessed by authorized personnel to ensure the security and integrity of data; and 3) a data auditing mechanism with the automatic creation of an Audit Trail to maintain a record of any creation, modification or deletion of instrument data.
Developed under the FDA standard for best practice ALCOA, Spectra Manager™ CFR provides secure access and features compliant with 21 CFR Part 11; system access requires a user name and password which are assigned by the Workgroup Manager and individual Access Levels determine the access to Administrative Tools which includes instrument configuration, analysis applications, user setup, workgroup setup and security policies as well as system and application history logs. Three levels of electronic signatures are required including creation, review, and approval stages. An audit trail is recorded in each data file recording any data processing performed on the spectral data.
System overview of Spectra Manager CFR
Features of Spectra Manager CFR
User Management
Based on the dual security category ([Access Level] and [Work Group]), it is possible to manage different authorization process in flexible and independent as total analysis systems, instrumentations and analytical applications.
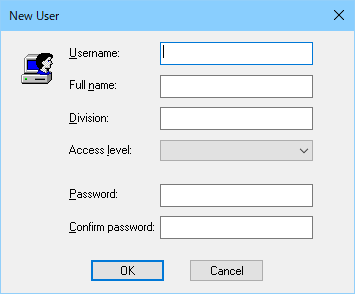
[New User] dialog box
User Account Security
Based on functions to prevent duplicate account or to protect password, and to prevent unauthorized access, administrative authorizations as system access and electric signature etc., can be managed strictly.
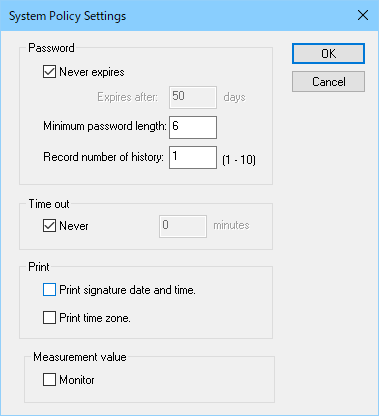
[System Policy Settings] dialog box
Audit Trail
It is categorized as 3 different records (system log, application log and data log), and it is recorded. Each log can be fi ltered and displayed under recorded date, user name etc, and it can be exported for audit trail review.
[Program Log] window
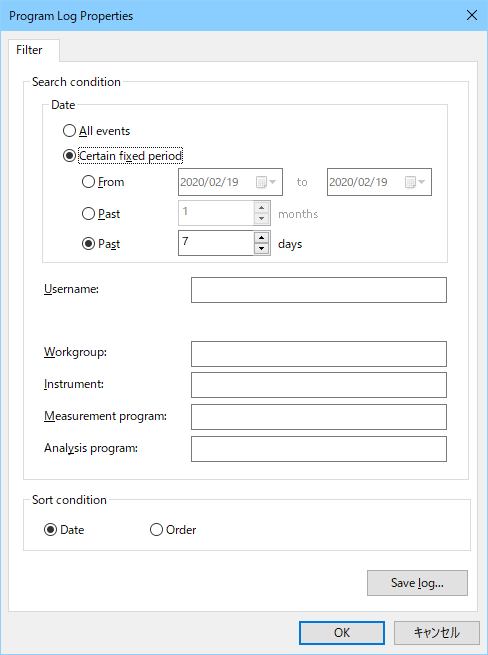
[Program Log Properties] dialog box
Enduring Electronic Record
Based on prohibiting function to delete electronic record and to overwrite save, and also functions for backup and restore data, electronic records can be saved properly and can be searched accurately during the data lifecycle.
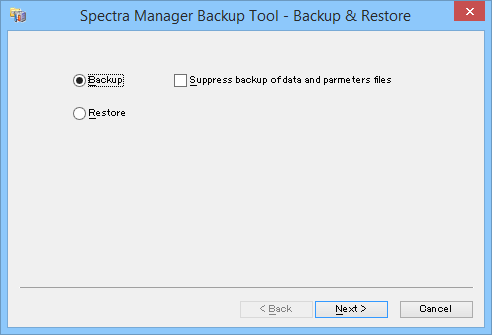
[Backup tool] program
Computerized System Validation
Computerized System Validation Spectra Manager CFR is developed and manufactured properly under quality control system adapted ISO 9001, and adapted CSV standard.