Introduction
Key Points
The validation function for the P-4000 series polarimeter offers inspections that meet the requirements of the United States, European, and Japanese pharmacopoeias (USP, EP, and JP).
Instrument inspection is essential for performing reliable measurements using analytical instruments. The P-4000 series polarimeters offer a validation function that enables efficient instrument inspections. Inspections that are required by USP, EP and JP are available, allowing easy compliance with each pharmacopoeia. This application note explains the inspection items required by the three pharmacopoeias and the validation function for the P-4000 series polarimeters.
Inspection items Required by USP/EP/JP
The items to inspect during a validation differ among pharmacopoeias. Table 1 shows the inspection items that are required by USP, EP, and JP as of May, 2025.
Table 1. Instrument inspections in pharmacopoeias
| Inspection items | Description in pharmacopoeias | ||
| USP-NF 2024, Issue 3 | EP11.0 (2022) | JP18 (2021) | |
| Temperature control | The temperature reading is within ±0.5ºC of that obtained using a temperature measurement device that is traceable to a National Institute of Standards and Technology (NIST) standard or equivalent. | Not mentioned | Not mentioned |
| Wavelength accuracy and bandwidth | For continuum light sources, recoding of the wavelength and bandwidth is recommended. For emission-line light sources, recording of the wavelength is recommended. | Not mentioned | Not mentioned |
| Accuracy of optical rotation | The measured optical rotation for the reference material (e.g., quartz plate) should agree with the stated certified value within the limits of the expanded uncertainty for the reference material added to the accuracy specification for the instrument. | Check the accuracy with certified quartz plates. Other certified materials such as sucrose solutions may also be used. | Check the accuracy using a sucrose solution. A quartz plate may also be used for daily verification. |
| Repeatability | Perform at least five replicate measurements of a reference material and calculate the standard deviation of the replicates. The result should be equal to or less than the stated repeatability specification for the instrument. | Not mentioned | Not mentioned |
| Linearity | The linearity should be checked when quantifying an enantiomer. The linearity is verified if three or more optical rotation values representing the operational range meet the criteria for accuracy. | When quantifying the amount of an enantiomer or the ratio of enantiomers, the linearity must be checked, for example using sucrose solutions. | Not mentioned |

Fig. 1. P-4000 series polarimeter
Validation Function for P-4000 Series Polarimeters
The P-4000 series polarimeters provide the inspections listed in Table 1. With the exception of “Wavelength Accuracy and Bandwidth”* in USP, users can perform each test using the validation function.
*“Wavelength Accuracy and Bandwidth” is not covered by the validation function, but records can be provided in written document form at the time of operational qualification (OQ). For more details, please contact us.
The validation function enables users to easily perform inspections compliant with the USP, EP, and JP pharmacopoeias. Test results can be saved as files readable by the P-4000 software and can also be printed or exported in text or CSV format.
- Inspection items compliant with USP/EP/JP
- Pass criteria prepared based on pharmacopoeias or instrument specifications
- Inspection parameter saving function, enabling retesting under identical conditions
- On-screen instructions to easily complete inspections
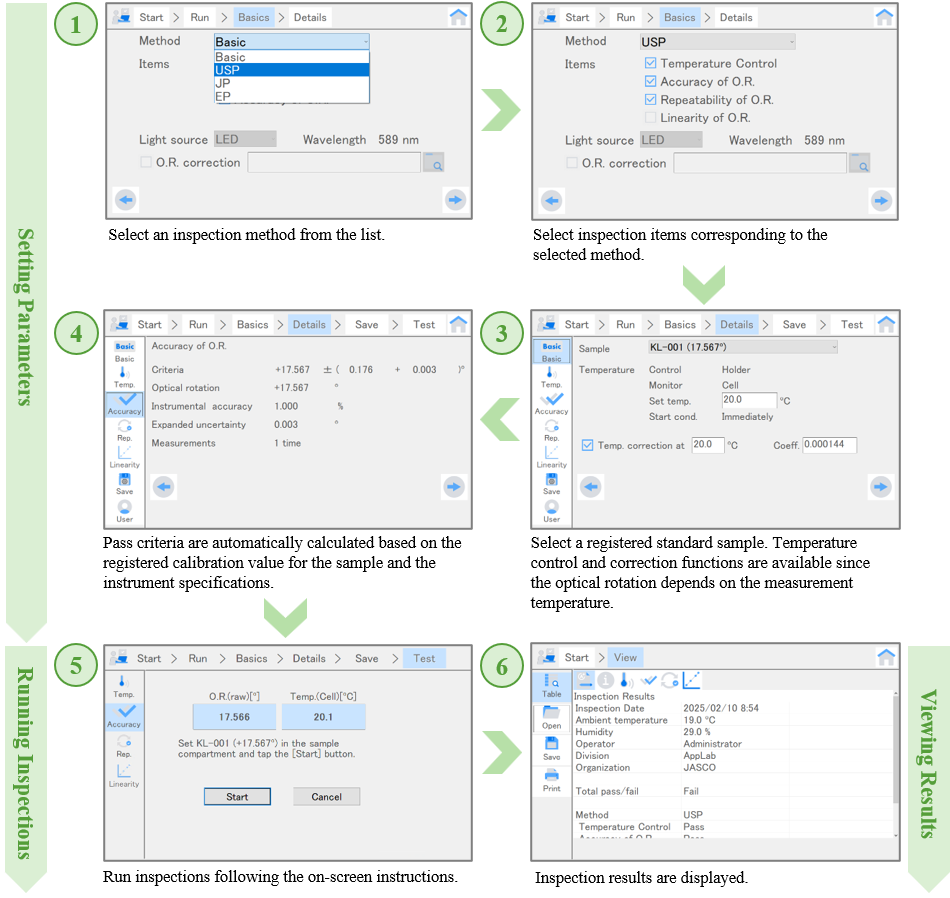
Fig. 2. Validation procedure for P-4000 series polarimeters
Keywords
Polarimeter, instrument inspection, validation, accuracy test, U.S. pharmacopoeia (USP), European pharmacopoeia (EP), Japanese pharmacopoeia (JP)
Conclusion
The P-4000 polarimeters provide a validation function that enables easy instrument inspections based on JP, EP, and USP.
References
1.Ministry of Health, Labour and Welfare: June 7, 2021, MHLW Ministerial Notification No. 220, “The Japanese Pharmacopoeia 18th edition”, (2021).
2.United States Pharmacopeial Convention: “USP-NF 2024 Issue 3”, (2024).
3.Council of Europe: “European Pharmacopoeia 11th edition”, (2023).