Introduction
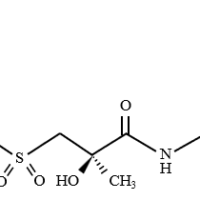
Rosuvastatin calcium is a drug newly listed in the 18th edition of the Japanese Pharmacopoeia (JP) and is used in the treatment of hypercholesterolemia. This drug competitively inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA reductase), which is involved in cholesterol synthesis in the liver.
In this report, we present the results of a system suitability evaluation for purity tests and assays of rosuvastatin calcium in accordance with the Japanese Pharmacopoeia, 18th Edition.

LC-4500 series HPLC system
Experimental
Instruments
Pump: PU-4580*
Autosampler: AS-4550
Column oven: CO-4060
UV detector: UV-4570
*with option units
Conditions
Column: Unifinepak C18 (3.0 mmI.D. x 150 mmL, 3 µm)
Eluent A: Water/acetonitrile/1 % trifluoroacetic acid (70/29/1)
Eluent B: Acetonitrile/water/1 % trifluoroacetic acid (75/24/1)
Gradient: A/B = 100/0 (0.0 min) ® 100/0 (30.0 min) ®
60/40 (50.00 min) ® 0/100 (60.0 min) ®
0/100 (70.0 min) ® 100/0 (70.1 min),
1cycle; 80 min
Flow rate: 0.75 mL/min
Column temp.: 40 ºC
Wavelength: 242 nm
Injection volume: 10 µL
Standard: Rosuvastatin calcium (Dissolve 35 mg with 12 mL acetonitrile and fill up to 50 mL with water.)
Keywords
Japanese Pharmacopoeia, rosuvastatin calcium, UV detector
Results
Structure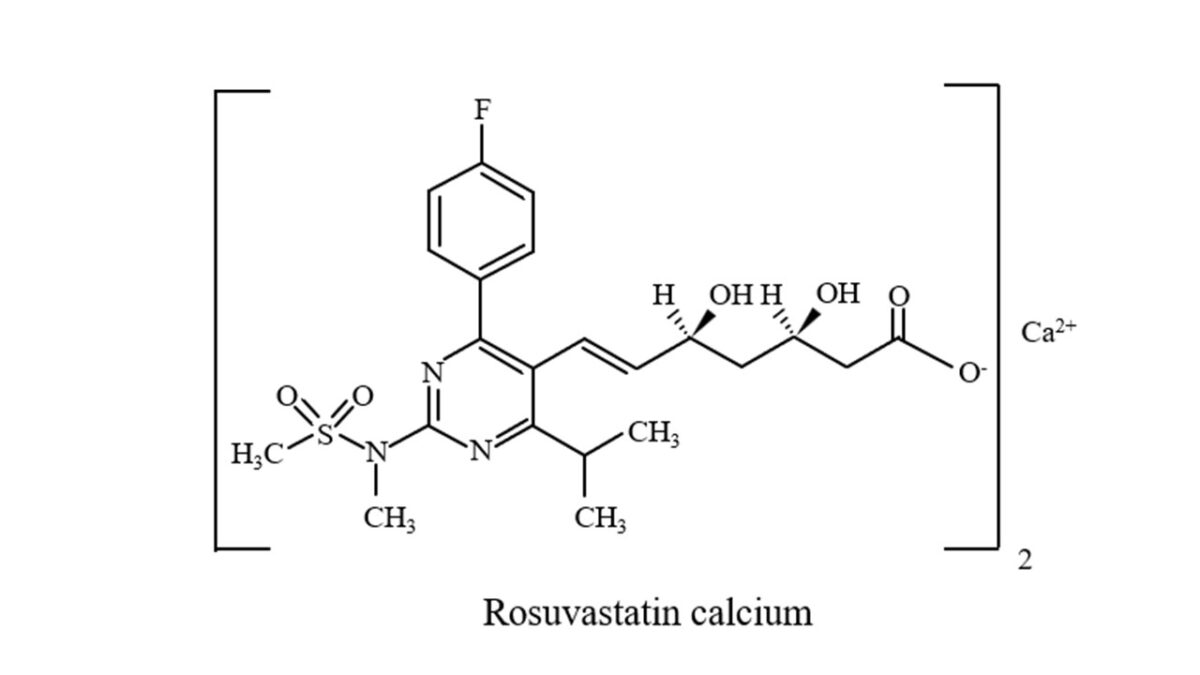
Results and Discussion
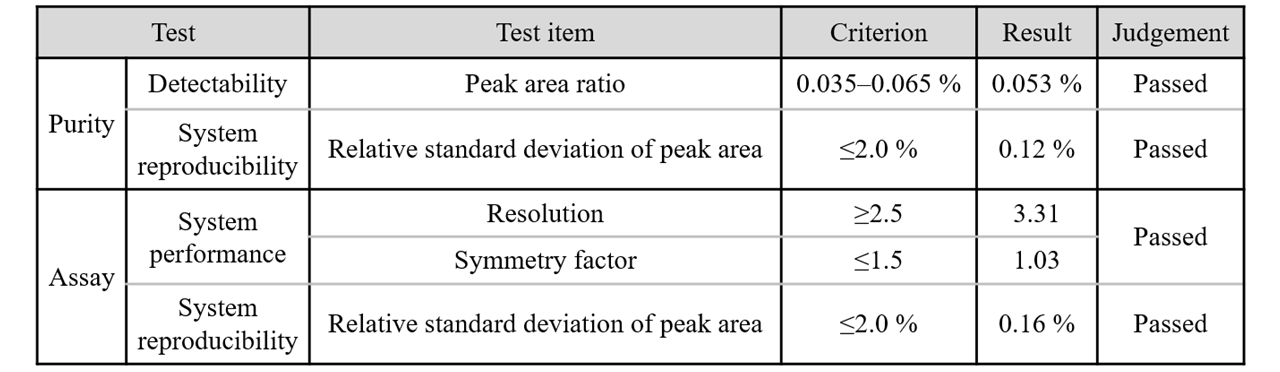
An overview of the system suitability for purity and assay tests on rosuvastatin calcium is shown in Table 1.
Table 1 Overview of system suitability for purity and assay tests on rosuvastatin calcium
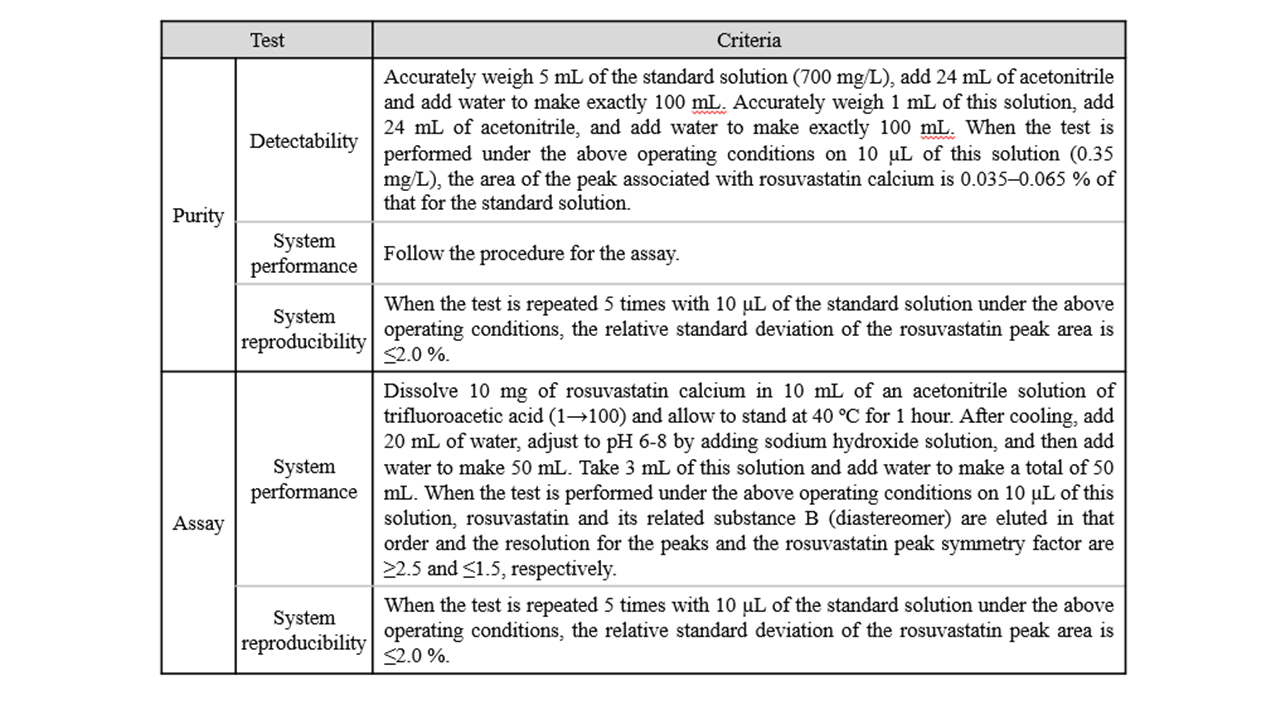
(1) Purity
Figure 1 shows the results of the detectability test. The ratio of the rosuvastatin peak area for the diluted solution (0.35 mg/L) to that for the standard solution (700 mg/L) was 0.053 %, which met the criterion of 0.035–0.065 %.
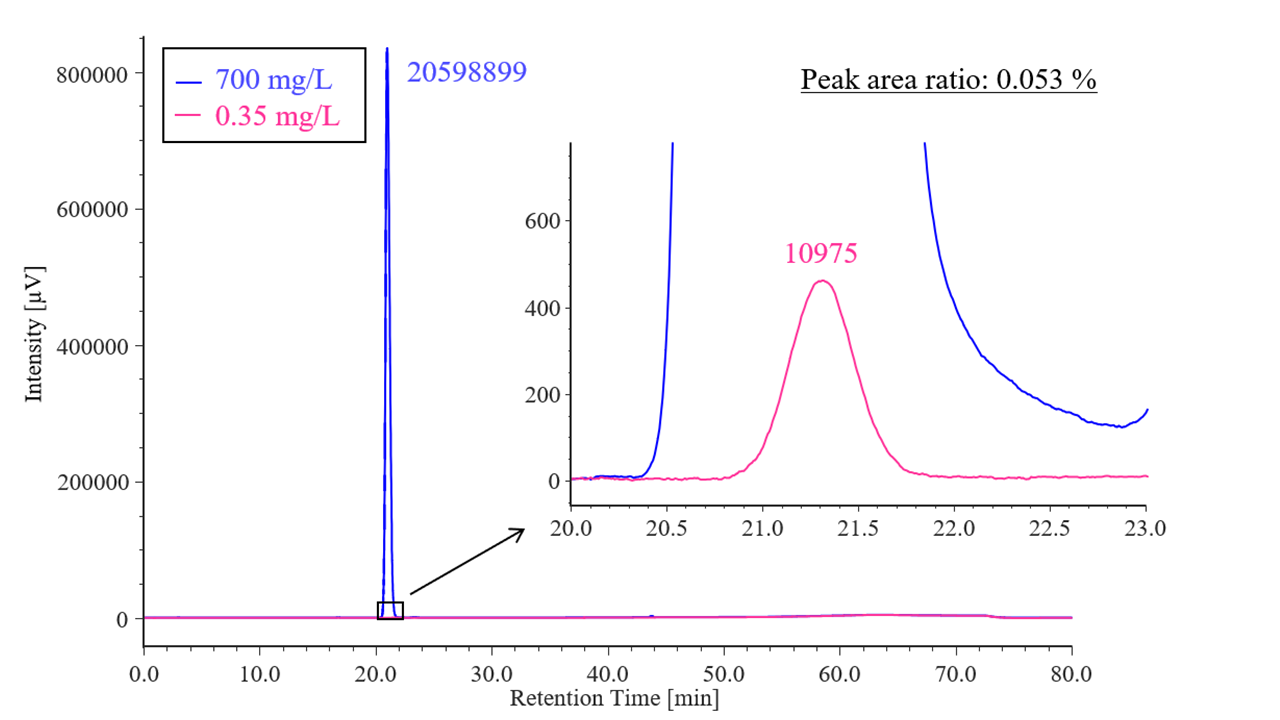
Fig. 1 Chromatogram of rosuvastatin for detectability test (700 mg/L, 0.35
Figure 2 shows chromatograms of rosuvastatin calcium standard solutions (n = 5), and Table 2 shows the results for peak area reproducibility based on the measured system reproducibility. The relative standard deviation of the peak area was 0.12 %, which met the criterion of ≤2.0 %.mg/L)
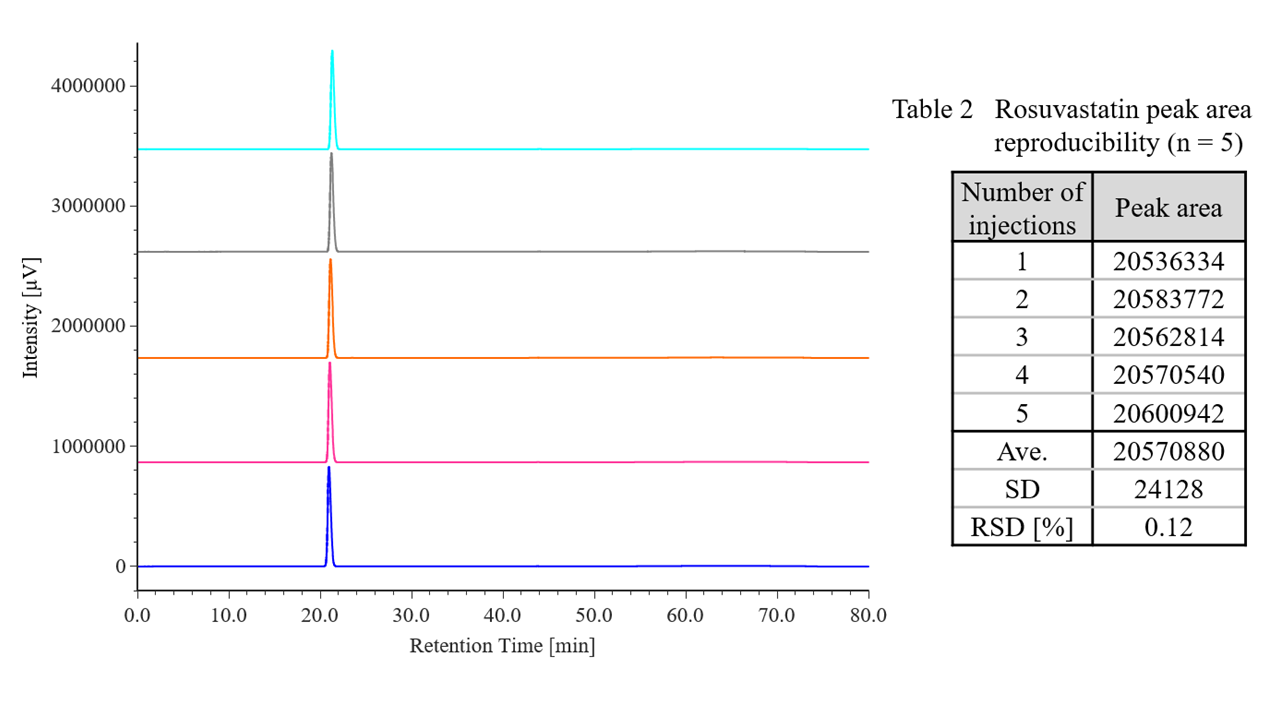
Fig. 2 Chromatograms of rosuvastatin calcium standard solutions (700 mg/L, n = 5)
(2) Assay
As confirmed by the system performance, Figure 3 shows a chromatogram of a solution of rosuvastatin and its related substance B, which were eluted in that order. The resolution for the peaks was 3.31 (criterion: ≥2.5), and the rosuvastatin peak symmetry factor was 1.03 (criterion: ≤1.5), both of which met the specified criteria.
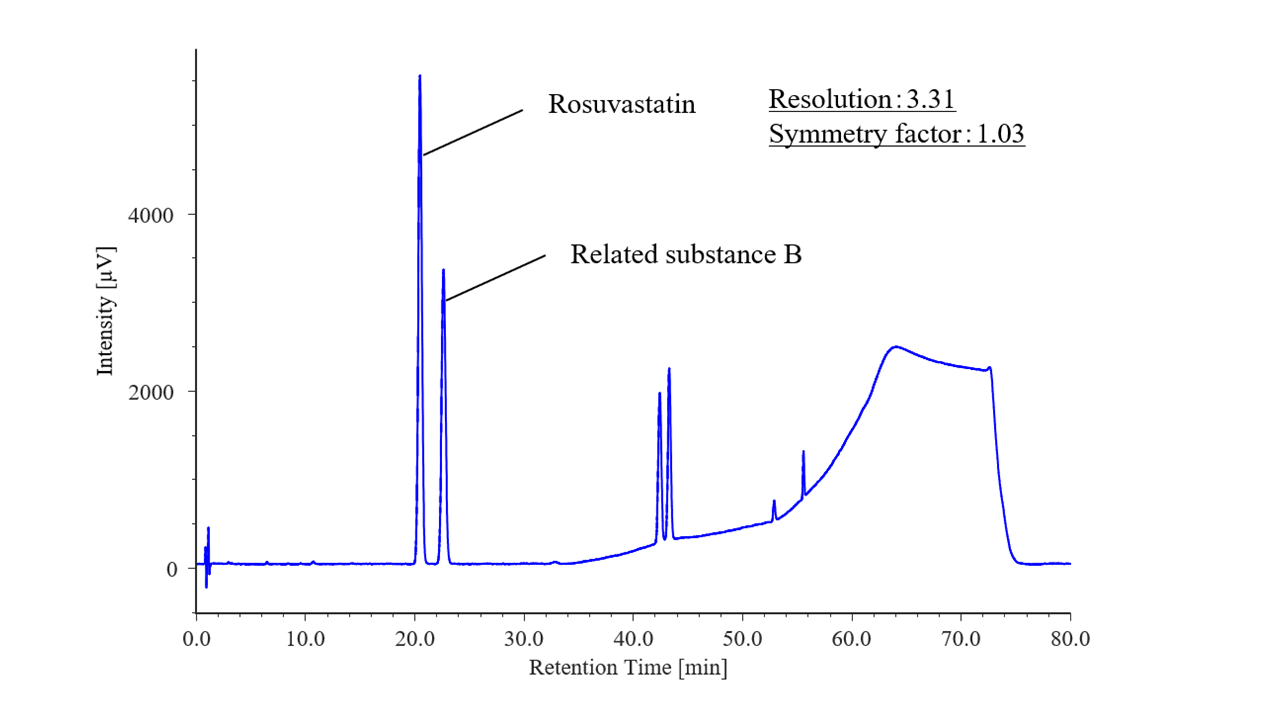
Fig. 3 Chromatogram of rosuvastatin and its related substance B
Figure 4 shows chromatograms of rosuvastatin calcium standard solutions (n = 5), and Table 3 shows the results for peak area reproducibility based on the measured system reproducibility. The relative standard deviation of the peak area was 0.16 %, which met the criterion of ≤2.0 %.
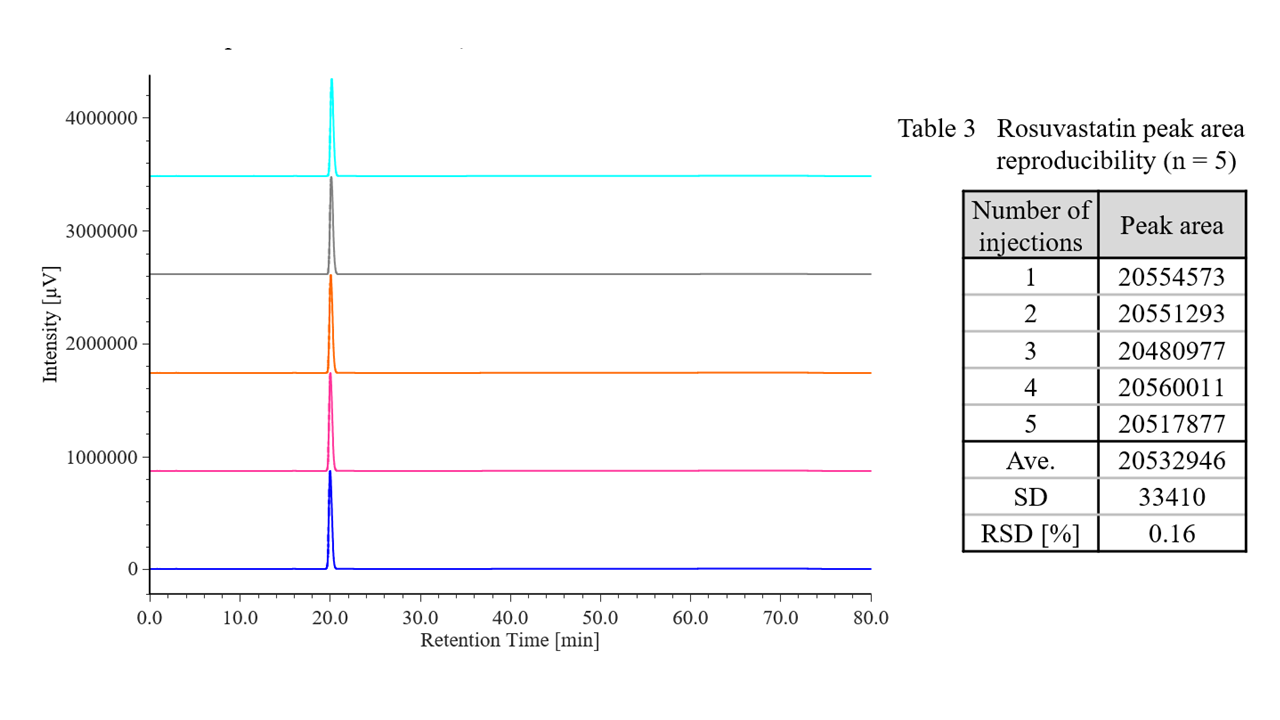
Fig. 4 Chromatograms of rosuvastatin calcium standard solutions (700 mg/L, n = 5)
Conclusion
We evaluated the system suitability for purity and assay tests of rosuvastatin calcium, which is newly listed in the 18th edition of the Japanese Pharmacopoeia. As shown in Table 3, all of the evaluation results met the specified criteria for the Japanese Pharmacopoeia.
Table 4 Results of system suitability evaluation for purity and assay tests of rosuvastatin calcium
Author: LC Technical Solution Group
A. Nakamura