Introduction
Celecoxib is a drug newly listed in the 18th edition of the Japanese Pharmacopoeia (JP). The drug selectively inhibits cyclooxygenase-2, an enzyme that synthesizes prostaglandins (PGs), which cause pain and inflammation, and suppresses PG synthesis, thereby relieving these symptoms.
In this report, we present the results of a system suitability evaluation for purity tests and assays of celecoxib in accordance with the Japanese Pharmacopoeia, 18th Edition.

LC-4500 series HPLC system
Experimental
Instruments
Pump: PU-4580
Degassing unit: DG-4580
Autosampler: AS-4550
Column oven: CO-4060
Detector: UV-4570
Conditions
Column: InertSustain Phenyl (4.6 mmI.D. x 250 mmL, 5 µm)
Eluent: 20 mM Sodium dihydrogen phosphate (adjusted to pH 3.0 with phosphoric acid), methanol, and acetonitrile (60:30:10)
Flow rate: 1.95 mL/min (adjusted so that the retention time of celecoxib is approximately 22 minutes)
Column temp.: 60 ºC
Preheating coil: 0.25 mmI.D. x 1000 mmL
Wavelength: 215 nm
Injection volume: 25 µL
Standard: Celecoxib (The dissolving and diluting solvent is in a mixture of methanol and water (3:1).)
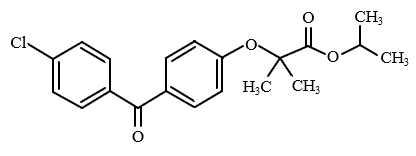
Structure
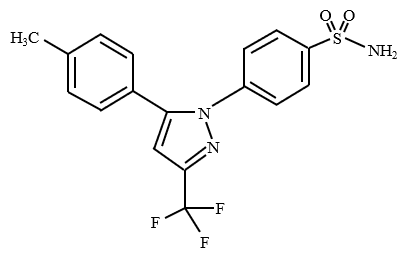
Celecoxib
Keywords
Japanese Pharmacopoeia, celecoxib, UV detector
Results
An overview of the system suitability for purity tests and assays of celecoxib is shown in Table 1.
Table 1 Overview of system suitability for purity tests and assays of celecoxib
| Test | Criteria | |
| Purity | Detectability | When the test is performed under the above operating conditions on 25 µL of a standard-purity solution (5 mg/L) diluted to 0.25 mg/L, the area of the peak associated with celecoxib is 3.5–6.5 % of that for the standard solution. |
| System performance | Follow the procedure for the assay. | |
| System reproducibility | ||
| Assay | System performance | When the test is performed under the above operating conditions on 25 µL of a standard-assay solution (500 mg/L), the number of theoretical plates and the peak symmetry factor for celecoxib are ≥6000 and ≤2.0, respectively. |
| System reproducibility | When the test is repeated 6 times with 25 µL of the standard-assay solution under the above operating conditions, the relative standard deviation of the celecoxib peak area is ≤1.0 %. | |
(1) Purity
Figure 1 shows the results of the detectability test. The ratio of the celecoxib peak area for the diluted solution (0.25 mg/L) to that for the standard solution (5 mg/L) was 5.0 %, which met the criterion of 3.5–6.5 %.
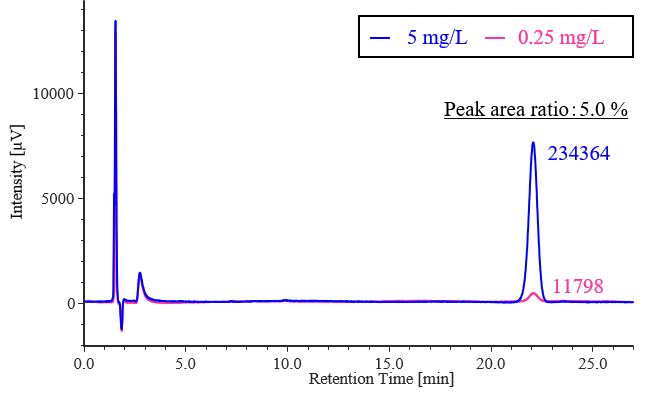
Fig. 1 Chromatogram of celecoxiib for the detectability test (5 mg/L, 0.25 mg/L)
(2) Assay
Figure 2 shows chromatograms of celecoxib standard solutions, and Table 2 shows the results for peak area reproducibility based on the measured system performance and system reproducibility. The number of theoretical plates was 12100 (criterion: ≥6000), the symmetry factor was 0.99 (criterion: ≤2.0), and the relative standard deviation of the peak area was 0.05 % (criterion: ≤1.0 %), all of which met the specified criteria.
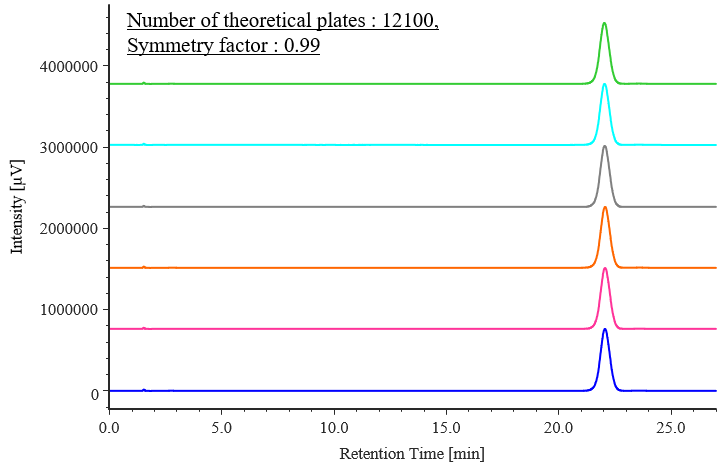
Fig. 2 Chromatograms of celecoxib standard solutions (500 mg/L, n = 6)
Table 2 Celecoxib peak area reproducibility (n = 6)
| Injection number | Peak area |
| 1 | 23140845 |
| 2 | 23143997 |
| 3 | 23139237 |
| 4 | 23162999 |
| 5 | 23135516 |
| 6 | 23157969 |
| Ave. | 23146760 |
| SD | 11090 |
| RSD[%] | 0.05 |
Conclusion
We evaluated the system suitability for purity tests and assays of celecoxib, which is newly listed in the 18th edition of the Japanese Pharmacopoeia. As shown in Table 3, all of the evaluation results met the specified criteria for the Japanese Pharmacopoeia.
Table 3 Results of system suitability evaluation for purity tests and assays of celecoxib
| Test | Test item | Criterion | Result | Judgement | |
| Purity | Detectability | Peak area ratio | 3.5–6.5 % | 5.0 % | Passed |
| Assay | System reproducibility | Number of theoretical plates | ≥6000 | 12100 | Passed |
| Symmetry factor | ≤2.0 | 0.99 | |||
| System reproducibility | Relative standard deviation of peak area | ≤1.0 % | 0.05 % | Passed | |